Wed, Apr 24, 2024
Volume 29, Issue 2 (4-2019)
JHNM 2019, 29(2): 90-96 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Poursafar Z, Jafroudi S, Baghaei M, Kazemnezhad Leyli E, Zarrizei M. Incidence and Extent of Bruising After Subcutaneous Injection of Enoxaparin Sodium in Patients Hospitalized at Coronary Care Units. JHNM 2019; 29 (2) :90-96
URL: http://hnmj.gums.ac.ir/article-1-764-en.html
URL: http://hnmj.gums.ac.ir/article-1-764-en.html
Zeynab Poursafar1 
 , Shirin Jafroudi *
, Shirin Jafroudi * 

 2, Mojgan Baghaei2
2, Mojgan Baghaei2 

 , Ehsan Kazemnezhad Leyli3
, Ehsan Kazemnezhad Leyli3 

 , Maryam Zarrizei1
, Maryam Zarrizei1 


 , Shirin Jafroudi *
, Shirin Jafroudi * 

 2, Mojgan Baghaei2
2, Mojgan Baghaei2 

 , Ehsan Kazemnezhad Leyli3
, Ehsan Kazemnezhad Leyli3 

 , Maryam Zarrizei1
, Maryam Zarrizei1 

1- Nursing (MSN), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
2- Instructor, Social Determinants of Health Research Center (SDHRC), Department of Nursing (Medical-Surgical), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
3- Associate Professor, Social Determinants of Health Research Center (SDHRC), Bio-Statistics, Guilan University of Medical Sciences, Rasht, Iran.
2- Instructor, Social Determinants of Health Research Center (SDHRC), Department of Nursing (Medical-Surgical), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
3- Associate Professor, Social Determinants of Health Research Center (SDHRC), Bio-Statistics, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 482 kb]
(1069 Downloads)
| Abstract (HTML) (3324 Views)
Full-Text: (1379 Views)
Introduction
Nurses play a unique role in using drugs for treatment, prevention and diagnosis of diseases, because they are responsible for administering drugs, monitoring their side-effects and make the medication program tolerable for the patients. This healthcare group should manage medication intervention to achieve the primary goal of drug therapy, i.e. maximizing the beneficial effects with minimal harm to the patient. The importance of this role of nurses is more prominent given the potential risk of drugs [1]. For example, enoxaparin sodium is injected subcutaneously in many patients admitted to hospitals, including those with ischemic heart disease [2], with the aim of preventing the progression of coronary artery bypass grafting [3] and its administration continues until discharge from hospital [4]. However, this drug has certain side effects such as subcutaneous bleeding, which is known to be the most common adverse effect of this drug [5, 6].
The reported incidence of 20.6% to 88.9% of ecchymosis following subcutaneous injection of this drug is a clear indication of such a complication [7]. Although bruising is a benign complication, it can cause discomfort to the patient [1]. In addition, this complication reduces the available space for subsequent injections, causes anxiety in the patient [5, 8], and hence, the likelihood of refusal from subsequent injections or discontinuation of treatment [5, 7, 9]. Thus, safe injection of a drug is one of the most important parts of drug administration [10]. In addition, subsequent evaluation of drug administration to determine the success of treatment is one of the most important aspects of drug therapy and an important nursing practice. Nurses should monitor patient’s response to drug therapy, including its therapeutic or adverse effects [11].
Because of high incidence of bruising or ecchymosis at the enoxaparin injection site [12], nurses must have the necessary skills to identify high-risk patients [13]. The patient’s individual characteristics can predispose him or her to side effects of drugs [14]. For example, Dadaeen [15] and Dehghani [16] reported a significant relationship between age and the extent of post-injection bruising, while Palese showed no association between age and bruising [7]. On the other hand, the gender variable is one of the characteristics that has a significant correlation with the extent of bruising after injection in the study of Dadaeen and women had experienced significantly higher bruise size than men [15]. However, Pourghaznein [9] found no significant relationship between gender and bruising, as well as body mass index and bruising. Regarding the importance of post-injection bruising and the contradictory findings in some of its related factors, this study attempted to investigate the incidence and extent of bruising after subcutaneous injection of enoxaparin sodium and its related factors.
Materials and Methods
This is an analytical cross-sectional study conducted on patients hospitalized at Coronary Care Unit (CCU) of Dr. Heshmat Educational and Treatment Center in Rasht, Iran in 2015. The patients were under treatment with enoxaparin sodium (6000 IU). The required sample size to determine the related factors to the incidence and extent of ecchymosis after subcutaneous injection of enoxaparin sodium with 95% confidence level was based on the study of Pourghaznein [9]. Because in this study, the reported incidence of bruising was 84.4% after 10-second injection method, and considering a 10% incidence accuracy, the sample size was obtained as 84.
The inclusion criteria were as follows: having had the prescribed order of enoxaparin sodium injection, being in the CCU within the first 24 hours of their admissions and ordered to stay at the hospital at least 48 hours after injection according to their therapists, not being under fibrinolytic therapy, angioplasty, or open surgery for coronary artery bypass grafting in the current admission period, having acceptable values for coagulation tests (Partial Thromboplastin Time [PTT], and International Normalized Ratio [INR] within the therapeutic range of 1.5-2 times the control level) according to the medical record, and finally, lacking any conditions like pregnancy or widespread skin lesions on the right side of the abdomen, and liver and blood diseases. On the other hand, the exclusion criteria were discharge earlier than the data collection period, death, and any change in the clinical condition that requires changing the drug dosage or its discontinuation such as emergency angiography or angioplasty.
Sampling started in June 30, 2015 and ended in February 2016 using consecutive sampling technique which lasted seven months. In this time period, 212 patients under treatment with enoxaparin sodium (6000 IU) were evaluated. Sampling was performed in the morning shift every day after approval by the Ethics Committee of Guilan University of Medical Sciences. Of the selected samples, some withdrew from the study (6 because of early discharge, 4 because of change in the drug dosage from 6000 to 8000 IU, and some because of discontinuation of drug injection by the physician, performing coronary angiography or death).
The relevant data were collected in this study by a two-part instrument. The first part was a questionnaire consisting of individual information (age, gender, waist circumference) and clinical information (Medical diagnosis, results of PTT and INR and platelet count, other diseases, and clinical risk factors) of the samples. The second part was the assessment of information related to the injection site with respect to bruising occurance and its extent. In this part, the data related to the incidence and size of bruise in the injection area, if present, were recorded (measured using a flexible transparent ruler). Content validity method was used to test the validity of the first section of the instrument. The second part just included the occurrence of the bruise and its extent measured by a flexible ruler, so it did not need evaluation with respect to its reliability.
The demographic and clinical information of the patients were obtained by referring to their medical files (Coagulation Tests, medical history) Measurement of waist circumference at its largest diameter was done using a centimeter tape. In order to standardize the injection method, one nurse as a member of research team performed all injections of enoxaparin sodium. The process involved disinfection of the injection site with 75% ethanol and drying the skin. The injection was performed once on the right side and above the navel (at a distance of at least 5 cm from the navel) in 10 seconds by a prefilled syringe with 0.6 mL solution vertically at 90° angle without aspiration, with the injection of 0.2 mL air in the syringe.
A single chronometer (Joerex stopwatch) was used to measure the injection duration. The selection of 10-second injection technique was according to Nair results where subcutaneous heparin injection time was in the range of 4 to 10 seconds by nurses [17]. After the completion of injection, the needle was removed at the same angle (90°) followed by a gentle pressure at the injection site without any massage. The injection area was marked by drawing a circle with a radius of 2.5 cm using a waterproof marker, and then the patient was provided with the necessary training to avoid massaging, scratching or touching the injection site. In addition, the nursing staff were told not to perform any subsequent injections in the upper abdomen of the patients along with the installation of the notice above the beds of samples. These action were planned to prevent any interference and error in the research results.
Another trained nurse from the research team assessed and measured (by a flexible ruler) the occurrence and extend of the bruising 48 hours after each injection. Choosing an interval of 48 hours to assess bruising occurrence was based on the study of Palese [7] who reported that the peak of bruising was at 48 hours after injection and begins to recover within 72 hours. The data related to the extent of bruising were classified according to Palese, in three categories: insignificant bruise (0-2 mm), small bruises (2-5 mm) and large bruises (>5 mm). The collected data were analyzed in SPSS V. 22 using descriptive (mean, standard deviation) and inferential (Chi-squared test, Independent t-test, and ANOVA) statistics. The Kolmogorov-Smirnov test was used to determine the normality of data distribution.
Results
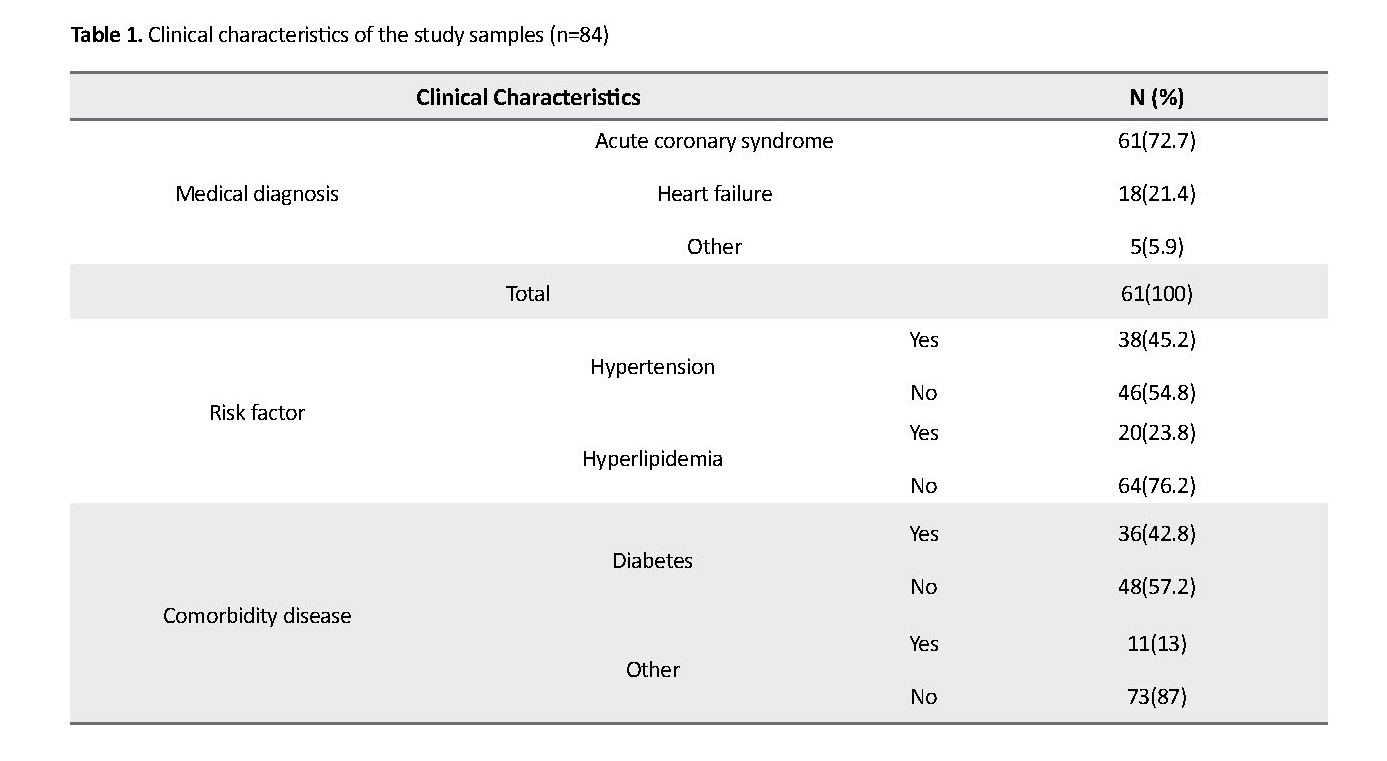
The majority (65.5%) of the study samples were women. The Mean±SD age of the samples was 66±11 years, and their Mean±SD waist circumference was 100.7±13.1 cm. The results showed the mean of platelet number (241286±81352), PTT (35.24±9.2) and INR (1.13±1.32). Table 1 presents the clinical characteristics of the participants.
Nurses play a unique role in using drugs for treatment, prevention and diagnosis of diseases, because they are responsible for administering drugs, monitoring their side-effects and make the medication program tolerable for the patients. This healthcare group should manage medication intervention to achieve the primary goal of drug therapy, i.e. maximizing the beneficial effects with minimal harm to the patient. The importance of this role of nurses is more prominent given the potential risk of drugs [1]. For example, enoxaparin sodium is injected subcutaneously in many patients admitted to hospitals, including those with ischemic heart disease [2], with the aim of preventing the progression of coronary artery bypass grafting [3] and its administration continues until discharge from hospital [4]. However, this drug has certain side effects such as subcutaneous bleeding, which is known to be the most common adverse effect of this drug [5, 6].
The reported incidence of 20.6% to 88.9% of ecchymosis following subcutaneous injection of this drug is a clear indication of such a complication [7]. Although bruising is a benign complication, it can cause discomfort to the patient [1]. In addition, this complication reduces the available space for subsequent injections, causes anxiety in the patient [5, 8], and hence, the likelihood of refusal from subsequent injections or discontinuation of treatment [5, 7, 9]. Thus, safe injection of a drug is one of the most important parts of drug administration [10]. In addition, subsequent evaluation of drug administration to determine the success of treatment is one of the most important aspects of drug therapy and an important nursing practice. Nurses should monitor patient’s response to drug therapy, including its therapeutic or adverse effects [11].
Because of high incidence of bruising or ecchymosis at the enoxaparin injection site [12], nurses must have the necessary skills to identify high-risk patients [13]. The patient’s individual characteristics can predispose him or her to side effects of drugs [14]. For example, Dadaeen [15] and Dehghani [16] reported a significant relationship between age and the extent of post-injection bruising, while Palese showed no association between age and bruising [7]. On the other hand, the gender variable is one of the characteristics that has a significant correlation with the extent of bruising after injection in the study of Dadaeen and women had experienced significantly higher bruise size than men [15]. However, Pourghaznein [9] found no significant relationship between gender and bruising, as well as body mass index and bruising. Regarding the importance of post-injection bruising and the contradictory findings in some of its related factors, this study attempted to investigate the incidence and extent of bruising after subcutaneous injection of enoxaparin sodium and its related factors.
Materials and Methods
This is an analytical cross-sectional study conducted on patients hospitalized at Coronary Care Unit (CCU) of Dr. Heshmat Educational and Treatment Center in Rasht, Iran in 2015. The patients were under treatment with enoxaparin sodium (6000 IU). The required sample size to determine the related factors to the incidence and extent of ecchymosis after subcutaneous injection of enoxaparin sodium with 95% confidence level was based on the study of Pourghaznein [9]. Because in this study, the reported incidence of bruising was 84.4% after 10-second injection method, and considering a 10% incidence accuracy, the sample size was obtained as 84.
The inclusion criteria were as follows: having had the prescribed order of enoxaparin sodium injection, being in the CCU within the first 24 hours of their admissions and ordered to stay at the hospital at least 48 hours after injection according to their therapists, not being under fibrinolytic therapy, angioplasty, or open surgery for coronary artery bypass grafting in the current admission period, having acceptable values for coagulation tests (Partial Thromboplastin Time [PTT], and International Normalized Ratio [INR] within the therapeutic range of 1.5-2 times the control level) according to the medical record, and finally, lacking any conditions like pregnancy or widespread skin lesions on the right side of the abdomen, and liver and blood diseases. On the other hand, the exclusion criteria were discharge earlier than the data collection period, death, and any change in the clinical condition that requires changing the drug dosage or its discontinuation such as emergency angiography or angioplasty.
Sampling started in June 30, 2015 and ended in February 2016 using consecutive sampling technique which lasted seven months. In this time period, 212 patients under treatment with enoxaparin sodium (6000 IU) were evaluated. Sampling was performed in the morning shift every day after approval by the Ethics Committee of Guilan University of Medical Sciences. Of the selected samples, some withdrew from the study (6 because of early discharge, 4 because of change in the drug dosage from 6000 to 8000 IU, and some because of discontinuation of drug injection by the physician, performing coronary angiography or death).
The relevant data were collected in this study by a two-part instrument. The first part was a questionnaire consisting of individual information (age, gender, waist circumference) and clinical information (Medical diagnosis, results of PTT and INR and platelet count, other diseases, and clinical risk factors) of the samples. The second part was the assessment of information related to the injection site with respect to bruising occurance and its extent. In this part, the data related to the incidence and size of bruise in the injection area, if present, were recorded (measured using a flexible transparent ruler). Content validity method was used to test the validity of the first section of the instrument. The second part just included the occurrence of the bruise and its extent measured by a flexible ruler, so it did not need evaluation with respect to its reliability.
The demographic and clinical information of the patients were obtained by referring to their medical files (Coagulation Tests, medical history) Measurement of waist circumference at its largest diameter was done using a centimeter tape. In order to standardize the injection method, one nurse as a member of research team performed all injections of enoxaparin sodium. The process involved disinfection of the injection site with 75% ethanol and drying the skin. The injection was performed once on the right side and above the navel (at a distance of at least 5 cm from the navel) in 10 seconds by a prefilled syringe with 0.6 mL solution vertically at 90° angle without aspiration, with the injection of 0.2 mL air in the syringe.
A single chronometer (Joerex stopwatch) was used to measure the injection duration. The selection of 10-second injection technique was according to Nair results where subcutaneous heparin injection time was in the range of 4 to 10 seconds by nurses [17]. After the completion of injection, the needle was removed at the same angle (90°) followed by a gentle pressure at the injection site without any massage. The injection area was marked by drawing a circle with a radius of 2.5 cm using a waterproof marker, and then the patient was provided with the necessary training to avoid massaging, scratching or touching the injection site. In addition, the nursing staff were told not to perform any subsequent injections in the upper abdomen of the patients along with the installation of the notice above the beds of samples. These action were planned to prevent any interference and error in the research results.
Another trained nurse from the research team assessed and measured (by a flexible ruler) the occurrence and extend of the bruising 48 hours after each injection. Choosing an interval of 48 hours to assess bruising occurrence was based on the study of Palese [7] who reported that the peak of bruising was at 48 hours after injection and begins to recover within 72 hours. The data related to the extent of bruising were classified according to Palese, in three categories: insignificant bruise (0-2 mm), small bruises (2-5 mm) and large bruises (>5 mm). The collected data were analyzed in SPSS V. 22 using descriptive (mean, standard deviation) and inferential (Chi-squared test, Independent t-test, and ANOVA) statistics. The Kolmogorov-Smirnov test was used to determine the normality of data distribution.
Results
The majority (65.5%) of the study samples were women. The Mean±SD age of the samples was 66±11 years, and their Mean±SD waist circumference was 100.7±13.1 cm. The results showed the mean of platelet number (241286±81352), PTT (35.24±9.2) and INR (1.13±1.32). Table 1 presents the clinical characteristics of the participants.
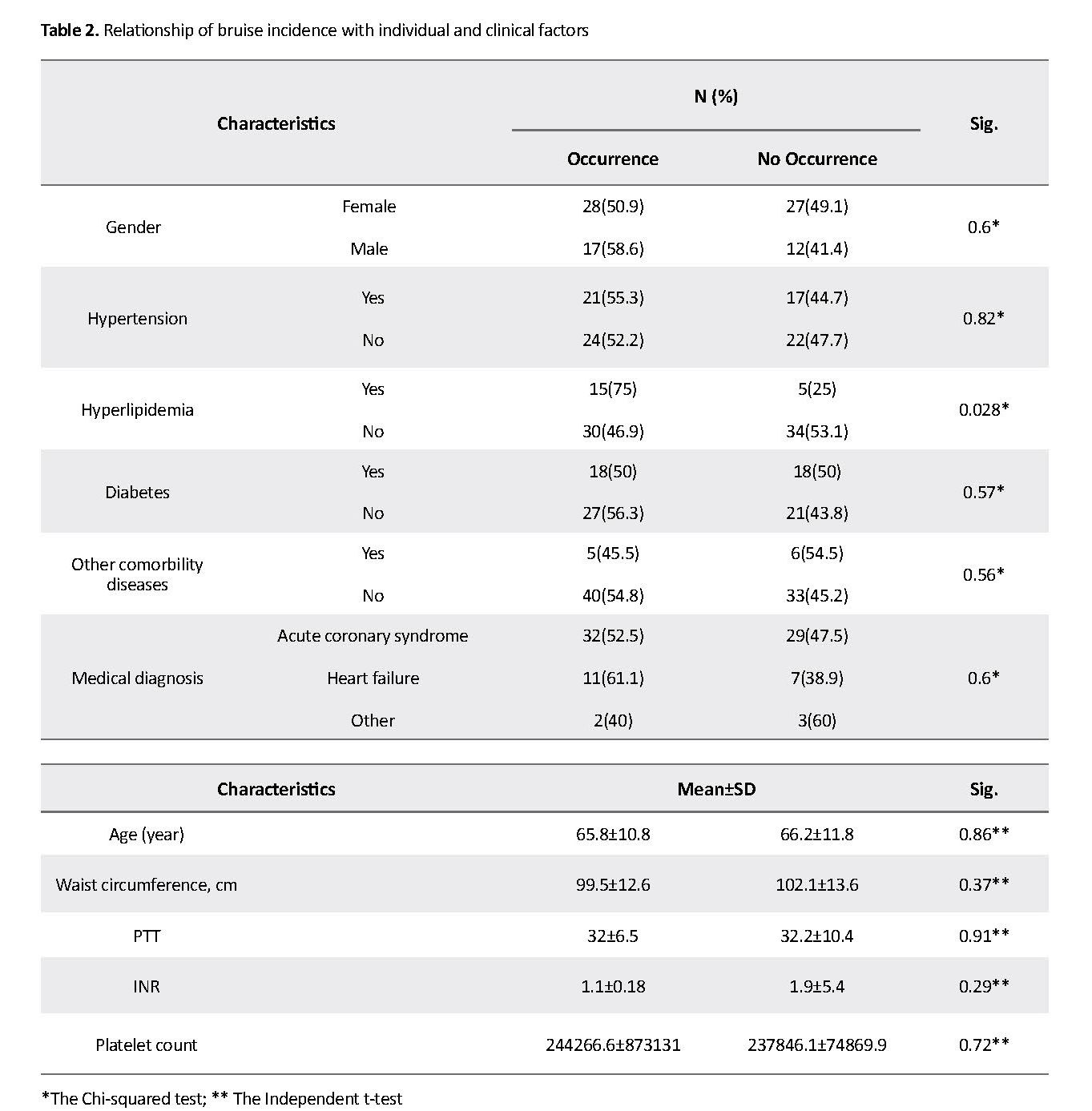
About 53.6% of the samples developed bruising after injection of enoxaparin sodium, while this complication did not occur in 46.4% of patients. Based on the results of the Chi-squared test, bruising had a significant relationship with hyperlipidemia as a risk factor (P=0.028) (Table 2). Moreover, 56% of the samples had insignificant bruises (0-2 mm), 23% had small bruises (2-5 mm), and 21% had large bruises (>5 mm). According to the Chi-squared test results, among risk factors, the bruise size had only a significant association with hypertension (P=0.048) (Table 3).
Discussion
Based on the study results, more than half of the samples developed bruises after injection of enoxaparin sodium. This finding indicates the need for paying special attention to this problem, because bruising can reduce the available space for subsequent injections, and cause mental and physical discomfort such as anxiety and body image disturbances [7]. In addition, the patient may refuse further injections or discontinue treatment due to loss of trust in the nurse’s competence [12]. Moreover, complications such as anxiety especially in patients with heart disease are important, because anxiety, by excessive stimulation of the sympathetic nervous system and releasing large amounts of catecholamines into the blood stream, can lead to vascular contraction, increased blood pressure and platelet activity, as well as dysrhythmia which have a deleterious effect on patients’ cardiovascular stability [18].
Based on the study results, more than half of the samples developed bruises after injection of enoxaparin sodium. This finding indicates the need for paying special attention to this problem, because bruising can reduce the available space for subsequent injections, and cause mental and physical discomfort such as anxiety and body image disturbances [7]. In addition, the patient may refuse further injections or discontinue treatment due to loss of trust in the nurse’s competence [12]. Moreover, complications such as anxiety especially in patients with heart disease are important, because anxiety, by excessive stimulation of the sympathetic nervous system and releasing large amounts of catecholamines into the blood stream, can lead to vascular contraction, increased blood pressure and platelet activity, as well as dysrhythmia which have a deleterious effect on patients’ cardiovascular stability [18].
Reviewing the literature indicates a lot of differences in the results related to the incidence of bruising. In the studies of Pourghaznein [9] and Balci Akpinar and Celebioglu [12], the incidence of bruising was considerably higher, while in the study of Palese [7], its rate was reported only 14.6% which is lower than the rate reported in the current study. These differences can be related to the difference in injection site, volume of injected drug, and definition of bruising. The volume of injected drug in this study was 0.6 mL and in the mentioned studies [9, 12], it was 0.4-4.5 mL. On the other hand, in our study bruising was defined as any change in color at the injection site within 48 hours after injection, while in the study of Palese it was defined as the presence of blood spots in an area of more than 2 mm [7]. Moreover, the findings of this study indicate the occurrence of large bruises in about one-fifth of the samples.
This result is promising in comparison with the results of Tehrani Neshat [19] where the incidence of large bruises was reported 65.4%. Such difference in outcome may be due to factors such as the angle of injection (90° vs. 45°), the site of injection (abdominal region in the current study and the arm in the study of Tehrani Neshat), and the scale of bruise size measurement (mm vs. mm²). The sample size in the study of Tehrani Neshat was also larger which can have an impact on the results of study.
Evaluation of the relationship between the incidence of bruising and individual and clinical factors showed that only hyperlipidemia had a statistically significant correlation with bruising. Thus, samples with hyperlipidemia were associated with a higher incidence of bruising compared to normal patients. This may be due to higher abdominal fat and the greater weight of patients with hyperlipidemia, since enoxaparin sodium is a hydrophilic drug that is dispensed into nonfat and plasma tissue [20]. In this way, the increase in blood fat can be associated with delayed drug dispersion leading to increased anticoagulant effects of the drug at the injection site and bruising.
A significant statistical relationship between hypertension and the extent of bruising is another finding of this study. This finding suggests that samples with hypertension, compared to those without this risk factor, had a higher incidence of bruises larger than 5 mm. This result can be attributed to the more capillary vulnerability in terms of hypertension that along with the anticoagulant effect of enoxaparin sodium, extends the size of bruises.
Overall, the findings of this study indicate a high incidence of bruising after subcutaneous injection of enoxaparin sodium and its significant association with the risk of hyperlipidemia, as well as a relatively large extent of bruising and its significant association with the risk factor of hypertension. Such findings can indicate that these two risk factors in patients with heart disease should be considered by caregivers, especially nurses, who should be well-trained in the diagnosis of high-risk patients. Their awareness of this important information in the pre-treatment phase helps to promote the safe and effective use of the drug and prevent its side effects [21]. Therefore, accurate evaluation of the patient’s condition and timely report of findings, and then planning appropriate measures to identify and control these factors, especially hypertension, in patients undergoing treatment with this drug, will be important in reducing the incidence of bruising. Considering that the present study was performed on patients with consecutive sampling in one hospital, generalization of the results to other patients is limited.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: 293003523).
Funding
This paper was extracted from a master thesis of first author in Guilan University of Medical Sciences. The research design was approved by the Social Determinants of Health Research Center at Guilan University of Medical Sciences.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Deputy of Social Determinants of Health Research Center at Guilan University of Medical Sciences, and all nurses in Dr. Heshmat Educational and Treatment Center.
Reference
Lehne RA. Pharmacology for nursing care. Amsterdam: Elsevier Health Sciences; 2013.
Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine: Section 1: Introduction to cardiovascular disorders [H Goudarzi Nejad, M Khodaei, S Razaghei, Persian trans.]. Tehran: Arjmand; 2012.
Dehghani Kh, Dehghani H, Najari Z. [Effect of subcutaneous enoxaparin injection duration on site-pain intensity in acute coronary syndrome patients hospitalized in CCU Afshar Hospital, Yazd, 2011 (Persian)]. Journal of Shahid Sadoughi University of Medical Sciences. 2012; 20(4):517-23.
Ayor JB, Griggs RC, Jey wing E. Cecillʼs Principles of internal medicine [M Es’hagh Hosseini, A Khalvat, Gh Derakhshan, H Samedanifar, A Abbaszade, M Gharounei, et al. Persian trans.]. Tehran: Arjmand; 2010.
Abdi K, Jouybari L, Sanagoo A, Shirafcan A, Batyar MM, Nasiri E, et al. [A study on the effect of the duration of subcutaneous heparin injection on bruising and pain of Panje Azar Hospital in Gorgan, 2008 (Persian)]. Journal of Gorgan Bouyeh Faculty of Nursing & Midwifery. 2011; 8(1):11-19.
Geng W, Zhang Y, Shi J. Comparison of modified versus conventional injection techniques of low molecular weight heparin in elderly. Pakistan Journal of Medical Sciences. 2018; 34(5):1142-5. [DOI:10.12669/pjms.345.15466] [PMID] [PMCID]
Palese A, Aidone E, Dante A, Pea F. Occurrence and extend of bruising according to duration of administration of subcutaneous low-molecular-weight heparin: Quasi-experimental case cross-over study. Journal of Cardiovascular Nursing. 2013; 28(5):473-82. [DOI:10.1097/JCN.0b013e3182578b87] [PMID]
Avsar G, Kasikci M. Assessment of four different methods in subcutaneous heparin application with regard to causing bruise and pain. International Journal of Nursing Practice. 2013; 19(4):402-8. [DOI:10.1111/ijn.12079] [PMID]
Pourghaznein T, Vahedian Azimi A, Asghari Jafarabadi M. The effect of injection duration and injection site on pain and bruising of subcutaneous injection of heparin. Journal of Clinical Nursing. 2014; 23(7-8):1105-13. [DOI:10.1111/jocn.12291] [PMID]
Potter PA, Perry GA, Stockert PA, Hall Am. Fundamentals of nursing. Maryland Heights, Missouri: Mosby; 2013.
Frandsen G, Penington SS. Abram’s clinical drug therapy: Rationales for nursing practice. Philadelphia: Williams & Wilkins; 2014.
Balci Akpinar R, Celebioglu A. Effect of injection duration on bruising associated with subcutaneous heparin: A quasi-experimental within-subject design. International Journal of Nursing Studies. 2007; 45(2008):812-7. [DOI:10.1016/j.ijnurstu.2007.02.005] [PMID]
Urden LD, Stacy KM, Lough ME. Critical care nursing: Diagnosis and management. Amsterdam: Elsevier Health Sciences; 2014.
Aschenbrenner DS, Venable SJ. Study guide for drug therapy in nursing. Philadelphia: Lippincott Williams & Wilkins; 2012.
Dadaeen A, Bahreini M, Bazi P, Ostovar A, Raeisei A, Dobaradaran S. The effect of duration of subcutaneous injection on the extent of bruising and pain intensity at injection sites among patients receiving enoxaparin Sodium: A randomized self-controlled clinical trial. International Cardiovascular Research Journal. 2015; 9(2):77-82.
Dehghani K, Najari Z, Dehghani H. Effect of subcutaneous enoxaparin injection duration on bruising size in acute coronary syndrome patients. Iranian Journal of Nursing and Midwifery Research. 2014; 19(6):564-68. [PMID] [PMCID]
Nair P, Kaur S, Sharma YP. Effect of time taken in injecting subcutaneous heparin injection with reference to site pain and bruising among patients receiving heparin therapy. Nursing and Midwifery Research Journal. 2008; 4(1):7-15.
Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Journal of Neuropsychiatric Disease and Treatment. 2010; 6:123-36. [PMCID] [PMID]
Tehrani Neshat B, Aziz Zade Forouzi M, Mohamad Alizade S. [Effect of subcutaneous heparin injection duration on bruising extend in patient hospitalization in Shiraz Hazrat Fateme and Shahid Beheshti hospitals (Persian)]. Journal of Shahid Sadoughei University of Medical Sciences. 2005; 12(4):86-94.
Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. British Journal of Clinical Pharmacology. 2003; 56(1):96-103. [DOI:10.1046/j.1365-2125.2003.01849.x] [PMID] [PMCID]
Karch AM. Focus on nursing pharmacology. Philadelphia: Lippincott Williams & Wilkins; 2013.
This result is promising in comparison with the results of Tehrani Neshat [19] where the incidence of large bruises was reported 65.4%. Such difference in outcome may be due to factors such as the angle of injection (90° vs. 45°), the site of injection (abdominal region in the current study and the arm in the study of Tehrani Neshat), and the scale of bruise size measurement (mm vs. mm²). The sample size in the study of Tehrani Neshat was also larger which can have an impact on the results of study.
Evaluation of the relationship between the incidence of bruising and individual and clinical factors showed that only hyperlipidemia had a statistically significant correlation with bruising. Thus, samples with hyperlipidemia were associated with a higher incidence of bruising compared to normal patients. This may be due to higher abdominal fat and the greater weight of patients with hyperlipidemia, since enoxaparin sodium is a hydrophilic drug that is dispensed into nonfat and plasma tissue [20]. In this way, the increase in blood fat can be associated with delayed drug dispersion leading to increased anticoagulant effects of the drug at the injection site and bruising.
A significant statistical relationship between hypertension and the extent of bruising is another finding of this study. This finding suggests that samples with hypertension, compared to those without this risk factor, had a higher incidence of bruises larger than 5 mm. This result can be attributed to the more capillary vulnerability in terms of hypertension that along with the anticoagulant effect of enoxaparin sodium, extends the size of bruises.
Overall, the findings of this study indicate a high incidence of bruising after subcutaneous injection of enoxaparin sodium and its significant association with the risk of hyperlipidemia, as well as a relatively large extent of bruising and its significant association with the risk factor of hypertension. Such findings can indicate that these two risk factors in patients with heart disease should be considered by caregivers, especially nurses, who should be well-trained in the diagnosis of high-risk patients. Their awareness of this important information in the pre-treatment phase helps to promote the safe and effective use of the drug and prevent its side effects [21]. Therefore, accurate evaluation of the patient’s condition and timely report of findings, and then planning appropriate measures to identify and control these factors, especially hypertension, in patients undergoing treatment with this drug, will be important in reducing the incidence of bruising. Considering that the present study was performed on patients with consecutive sampling in one hospital, generalization of the results to other patients is limited.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: 293003523).
Funding
This paper was extracted from a master thesis of first author in Guilan University of Medical Sciences. The research design was approved by the Social Determinants of Health Research Center at Guilan University of Medical Sciences.
Authors contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Deputy of Social Determinants of Health Research Center at Guilan University of Medical Sciences, and all nurses in Dr. Heshmat Educational and Treatment Center.
Reference
Lehne RA. Pharmacology for nursing care. Amsterdam: Elsevier Health Sciences; 2013.
Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine: Section 1: Introduction to cardiovascular disorders [H Goudarzi Nejad, M Khodaei, S Razaghei, Persian trans.]. Tehran: Arjmand; 2012.
Dehghani Kh, Dehghani H, Najari Z. [Effect of subcutaneous enoxaparin injection duration on site-pain intensity in acute coronary syndrome patients hospitalized in CCU Afshar Hospital, Yazd, 2011 (Persian)]. Journal of Shahid Sadoughi University of Medical Sciences. 2012; 20(4):517-23.
Ayor JB, Griggs RC, Jey wing E. Cecillʼs Principles of internal medicine [M Es’hagh Hosseini, A Khalvat, Gh Derakhshan, H Samedanifar, A Abbaszade, M Gharounei, et al. Persian trans.]. Tehran: Arjmand; 2010.
Abdi K, Jouybari L, Sanagoo A, Shirafcan A, Batyar MM, Nasiri E, et al. [A study on the effect of the duration of subcutaneous heparin injection on bruising and pain of Panje Azar Hospital in Gorgan, 2008 (Persian)]. Journal of Gorgan Bouyeh Faculty of Nursing & Midwifery. 2011; 8(1):11-19.
Geng W, Zhang Y, Shi J. Comparison of modified versus conventional injection techniques of low molecular weight heparin in elderly. Pakistan Journal of Medical Sciences. 2018; 34(5):1142-5. [DOI:10.12669/pjms.345.15466] [PMID] [PMCID]
Palese A, Aidone E, Dante A, Pea F. Occurrence and extend of bruising according to duration of administration of subcutaneous low-molecular-weight heparin: Quasi-experimental case cross-over study. Journal of Cardiovascular Nursing. 2013; 28(5):473-82. [DOI:10.1097/JCN.0b013e3182578b87] [PMID]
Avsar G, Kasikci M. Assessment of four different methods in subcutaneous heparin application with regard to causing bruise and pain. International Journal of Nursing Practice. 2013; 19(4):402-8. [DOI:10.1111/ijn.12079] [PMID]
Pourghaznein T, Vahedian Azimi A, Asghari Jafarabadi M. The effect of injection duration and injection site on pain and bruising of subcutaneous injection of heparin. Journal of Clinical Nursing. 2014; 23(7-8):1105-13. [DOI:10.1111/jocn.12291] [PMID]
Potter PA, Perry GA, Stockert PA, Hall Am. Fundamentals of nursing. Maryland Heights, Missouri: Mosby; 2013.
Frandsen G, Penington SS. Abram’s clinical drug therapy: Rationales for nursing practice. Philadelphia: Williams & Wilkins; 2014.
Balci Akpinar R, Celebioglu A. Effect of injection duration on bruising associated with subcutaneous heparin: A quasi-experimental within-subject design. International Journal of Nursing Studies. 2007; 45(2008):812-7. [DOI:10.1016/j.ijnurstu.2007.02.005] [PMID]
Urden LD, Stacy KM, Lough ME. Critical care nursing: Diagnosis and management. Amsterdam: Elsevier Health Sciences; 2014.
Aschenbrenner DS, Venable SJ. Study guide for drug therapy in nursing. Philadelphia: Lippincott Williams & Wilkins; 2012.
Dadaeen A, Bahreini M, Bazi P, Ostovar A, Raeisei A, Dobaradaran S. The effect of duration of subcutaneous injection on the extent of bruising and pain intensity at injection sites among patients receiving enoxaparin Sodium: A randomized self-controlled clinical trial. International Cardiovascular Research Journal. 2015; 9(2):77-82.
Dehghani K, Najari Z, Dehghani H. Effect of subcutaneous enoxaparin injection duration on bruising size in acute coronary syndrome patients. Iranian Journal of Nursing and Midwifery Research. 2014; 19(6):564-68. [PMID] [PMCID]
Nair P, Kaur S, Sharma YP. Effect of time taken in injecting subcutaneous heparin injection with reference to site pain and bruising among patients receiving heparin therapy. Nursing and Midwifery Research Journal. 2008; 4(1):7-15.
Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Journal of Neuropsychiatric Disease and Treatment. 2010; 6:123-36. [PMCID] [PMID]
Tehrani Neshat B, Aziz Zade Forouzi M, Mohamad Alizade S. [Effect of subcutaneous heparin injection duration on bruising extend in patient hospitalization in Shiraz Hazrat Fateme and Shahid Beheshti hospitals (Persian)]. Journal of Shahid Sadoughei University of Medical Sciences. 2005; 12(4):86-94.
Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. British Journal of Clinical Pharmacology. 2003; 56(1):96-103. [DOI:10.1046/j.1365-2125.2003.01849.x] [PMID] [PMCID]
Karch AM. Focus on nursing pharmacology. Philadelphia: Lippincott Williams & Wilkins; 2013.
Article Type : Research |
Subject:
Special
Received: 2018/07/25 | Accepted: 2018/10/18 | Published: 2019/04/1
Received: 2018/07/25 | Accepted: 2018/10/18 | Published: 2019/04/1
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |