Tue, Feb 3, 2026
Volume 35, Issue 2 (3-2025)
JHNM 2025, 35(2): 91-97 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Vanda R, Moghadam-Sangcholi M, Taghavi S, Sadeghi H, Bazarganipour F. Effect of Probiotics on Pregnancy Outcomes in Pregnant Women Receiving 17α-OHP Injection: A Randomized Clinical Trial. JHNM 2025; 35 (2) :91-97
URL: http://hnmj.gums.ac.ir/article-1-2379-en.html
URL: http://hnmj.gums.ac.ir/article-1-2379-en.html
Raziyeh Vanda1 

 , Mansoureh Moghadam-Sangcholi1
, Mansoureh Moghadam-Sangcholi1 

 , Seyed-Abdolvahab Taghavi2
, Seyed-Abdolvahab Taghavi2 

 , Hossein Sadeghi3
, Hossein Sadeghi3 

 , Fatemeh Bazarganipour *4
, Fatemeh Bazarganipour *4 




 , Mansoureh Moghadam-Sangcholi1
, Mansoureh Moghadam-Sangcholi1 

 , Seyed-Abdolvahab Taghavi2
, Seyed-Abdolvahab Taghavi2 

 , Hossein Sadeghi3
, Hossein Sadeghi3 

 , Fatemeh Bazarganipour *4
, Fatemeh Bazarganipour *4 


1- MD, Department of Gynecology and Obstetrics, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran.
2- PhD, Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
3- PhD, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
4- PhD, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran. ,f.bazarganipour@gmail.com
2- PhD, Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
3- PhD, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran.
4- PhD, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran. ,
Full-Text [PDF 593 kb]
(596 Downloads)
| Abstract (HTML) (1026 Views)
Full-Text: (463 Views)
Introduction
Preterm Birth (PTB) is defined as birth before 37 weeks of gestation [1]. Up to 50% of PTBs are associated with maternal infection [2]. Genitourinary infections, including urinary tract infections, bacterial vaginosis and yeast vaginitis, annually affect about one billion women in the world. In recent years, genitourinary infections have been an important risk factor for PTB [3]. The most common route for urogenital pathogens that cause preterm labor is the ascending pathway [4]. Proteolytic enzymes act directly on the collagen of the cervix and fetal membranes and lead to early thinning of the cervix and its insufficiency, weakening of the fetal membranes, and subsequently Preterm premature rupture of membranes (PPROM) [5].
Probiotics, as living microorganisms, can provide health benefits to the host when administered in sufficient amounts. Probiotics displace and kill pathogens, and modulate the immune response by interfering with the inflammatory cascades that lead to PTB [6]. The mechanism of action of probiotics in the vagina is probably multifactorial. The production of lactic acid, bacteriocins, and hydrogen peroxide and the immune response modulation can be the possible mechanisms [7].
A cohort study in Norway reported a significant protective effect against spontaneous PTB in women who had a high intake of probiotic milk [8]. A review study comparing probiotics with placebo reported no statistically significant difference in gestational age at birth [9]. Another review study reported no significant finding that probiotics increased or decreased the incidence of PTB [10]. Regarding the effect of probiotic oral supplementation in pregnancy on the risk of PTB, no benefit or harm has been reported, and more studies are needed in this field. However, it has been proposed that the combination of L. acidophilus and Bifidobacterium bifidum probiotic species may be more useful in improving pregnancy outcomes [11]. Progesterone has been shown to suppress the contractions of the myometrium; therefore, one strategy is the use of supplemental progestogens, including Intramuscular (IM) injection of 17α-hydroxyprogesterone caproate (17α-OHP) in women with a singleton pregnancy and a history of singleton spontaneous PTB [12]. The 17α-OHP has been recommended to prevent PTB by the American College of Obstetricians and Gynecologists (ACOG) and the society for maternal-fetal medicine (SMFM). In August 2021, ACOG recommended that women with a singleton pregnancy and a history of spontaneous PTB should receive progesterone supplementation vaginally or by IM injection [13]. However, considering the consequences of PTB and the lack of theoretical agreement on the use of different drugs for the prevention of PTB, it is appropriate to conduct further studies in this field. Due to the existing contradictions regarding the effects of probiotics in women at high risk of PTB and considering the benefits of probiotics (bacteriotherapy and immune regulation), this study aims to investigate the efficacy of adjuvant administration of probiotics on the spontaneous PTB and the related pregnancy outcomes in pregnant women at high risk for PTB receiving 17α-OHP.
Materials and Methods
This is a randomized clinical trial in compliance with the CONSORT guidelines [14]. The study population consists of all pregnant women referred to the gynecology clinics in Yasuj, Iran, from 2020 to 2022 who were receiving 17α-OHP injection (250 mg, intramuscular injection [IM]). Using the related formula, and by considering α=0.05, β=0.80, a 15% sample dropout rate, 20% cesarean rate in the probiotic group, and 46.7% cesarean rate in the control group according to the findings of Badehnoosh et al. [15], the sample size was determined to be 60 per group. First, 132 participants were assessed for eligibility, of whom 12 were excluded due to not meeting inclusion criteria and declined to participate. Finally, 120 patients were included. Inclusion criteria were a history of high-risk PTB (including a history of PTB or pregnancy termination at the second trimester), willingness to participate in the study, age 18-45 years, gestational age 16-24 weeks, no syphilis, gonorrhea or HIV, no elective or emergency cervical cerclage, and no maternal insulin-dependent diabetes mellitus, hypertension, lupus and clinical chorioamnionitis based on medical records. Exclusion criteria were unwillingness to continue participation in the study, failure to complete the treatment, taking drugs that affect the intestinal microbial flora (such as antibiotics), occurrence of any genital or urinary tract infection that required antibiotic treatment during the trial, having a fetus with congenital malformations and abnormal scan anomalies, or clinical chorioamnionitis.
Eligible participants were assigned to two groups: Group A: Lactofem capsule (containing Lactobacillus acidophilus 2×109 cfu/g, Bifidobacterium bifidus 2×109 cfu/g, Lactobacillus rutri 2×109 cfu/g, Lactobacillus fermentum 2×109 cfu/g; bio-capsule weight of 500 mg made by Zist Takhmir Company, Iran, administered orally and daily from the 16th to the 37th week of pregnancy) and Group B: Placebo capsules containing starch powder (not harmful during pregnancy) prepared by the medicinal plants laboratory, Yasuj University of Medical Sciences. Capsules in two groups were similar in shape and package. The randomization of patients was performed with a random allocation software using the block randomization method. To hide the treatment options, the list of treatments was placed in sealed and numbered envelopes (in a sequencing order). Participants and physicians were blinded to the allocation and were not aware of group allocation.
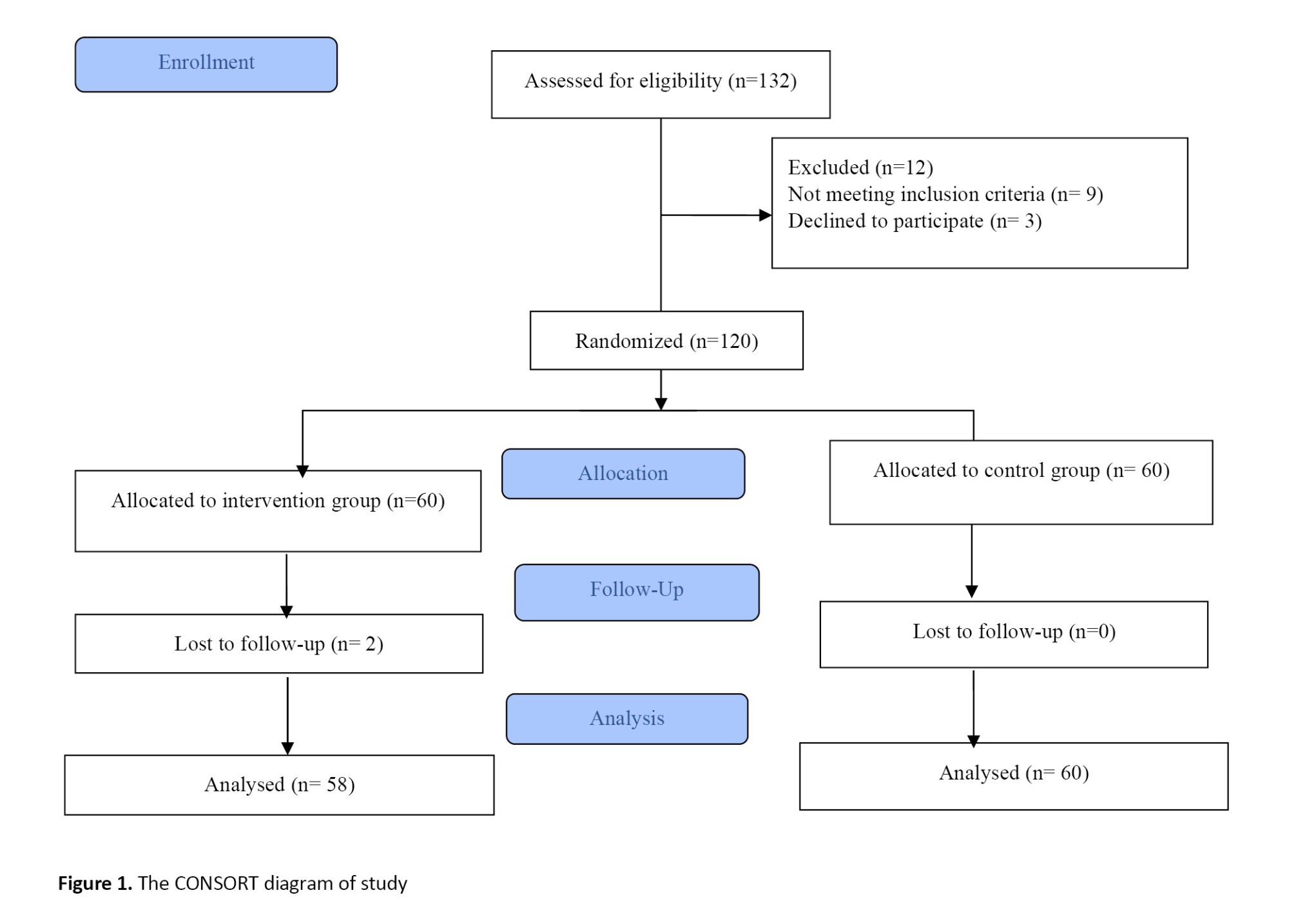
Socio-demographic and reproductive information including age, body mass index (BMI), educational level, occupation (housewife or employed), gravidity, history of abortion and parity were first recorded. All participants were informed that they could leave the study at any time. All participants followed the prenatal care and pregnancy outcome in Shahid Mofatteh gynecology clinic under the supervision of one gynecologist. Obstetric information included the history of PPROM, preterm labor (late and early) and mode of delivery (Instrumental, cesarean section, or normal vaginal delivery [NVD]). Neonatal information included weight, height and head circumference of the newborn and the Apgar score (1 and 5 minutes after birth). The side effects including fever, itching, diarrhea, vomiting, or other gastrointestinal symptoms were also recorded. PTB was considered as the primary outcome and pregnancy-related complications as the secondary outcome (Figure 1).
Data analysis was carried out in SPSS software, version 21 using descriptive statistics (frequency, percent, Mean±SD), chi-square test and independent t-test. Kolmogorov–Smirnov test was used to examine the normality of data distribution. The significance level was set at 0.05. There were no missing data. Therefore, no missing imputation technique was used.
Results
Two women from the probiotic group left the study because they were unwilling to continue the treatment. Therefore, the data of 118 women was analyzed. Their characteristics are presented in Table 1.
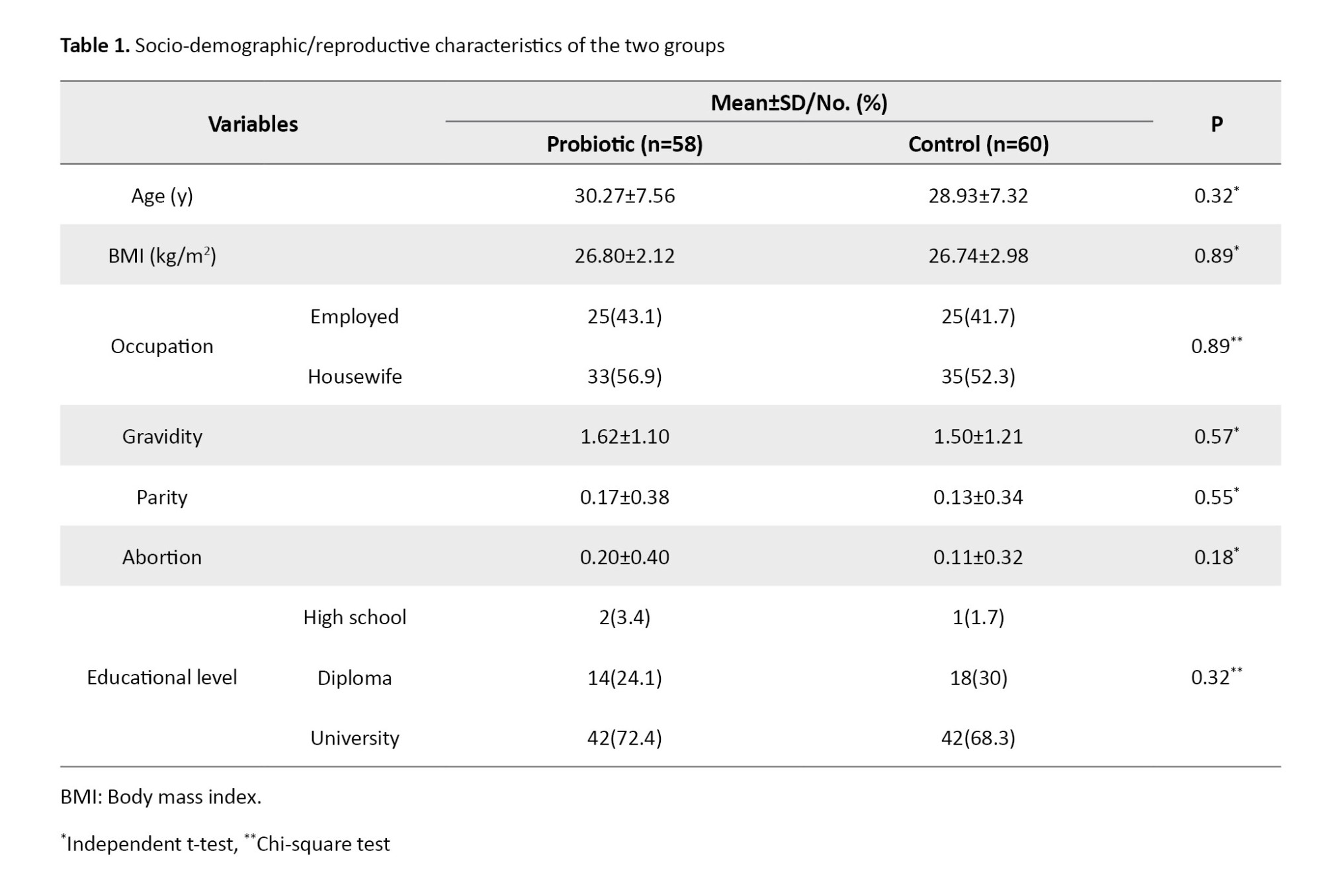
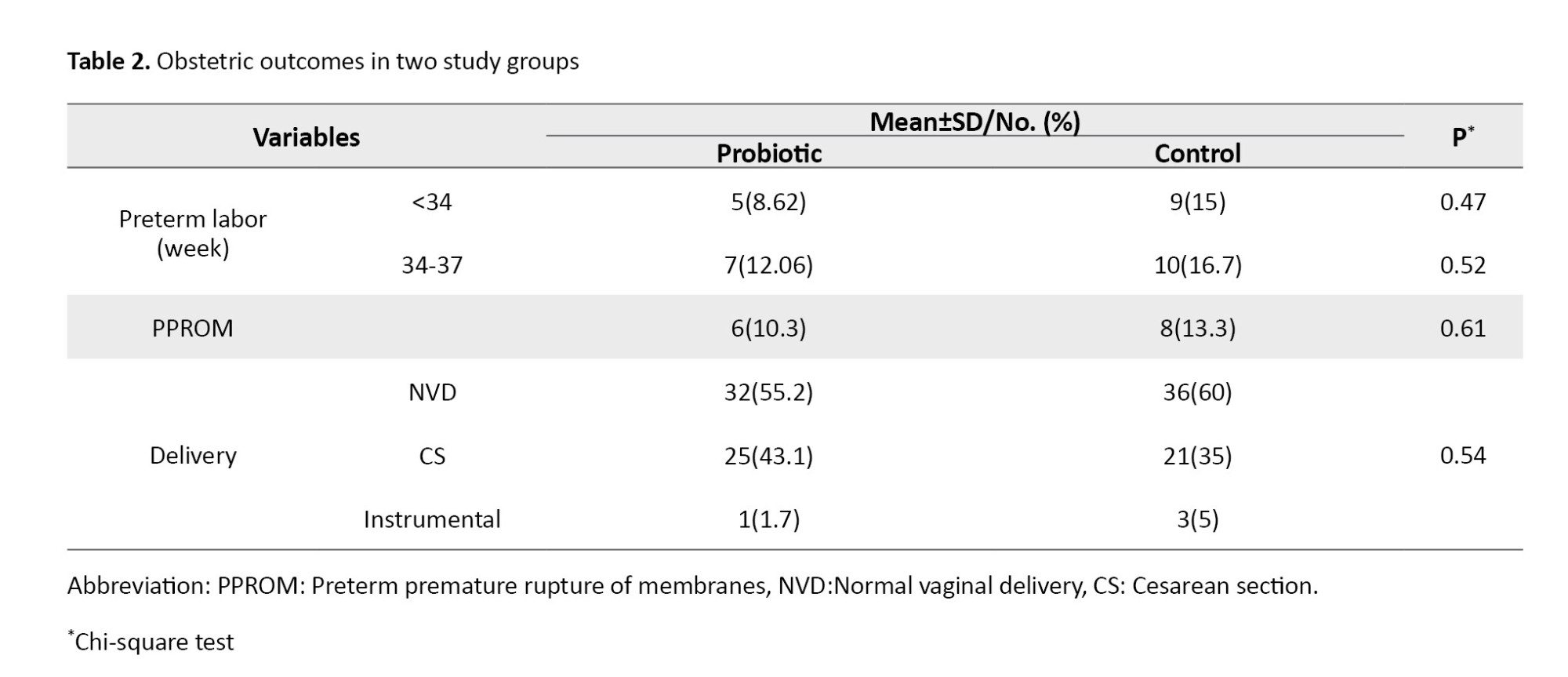
There were no significant differences between the two groups in terms of age, BMI, educational level, occupation, gravidity, abortion, or parity. Table 2 presents the obstetric information for the two groups. As can be seen, 8.62% and 15% of women in the probiotic and placebo groups had PTB before the 34th week of pregnancy, while 12.06% and 16.7% had PTB from the 34th to the 37th week of pregnancy, respectively. Results also showed no significant differences between the two groups regarding PPROM or mode of delivery.
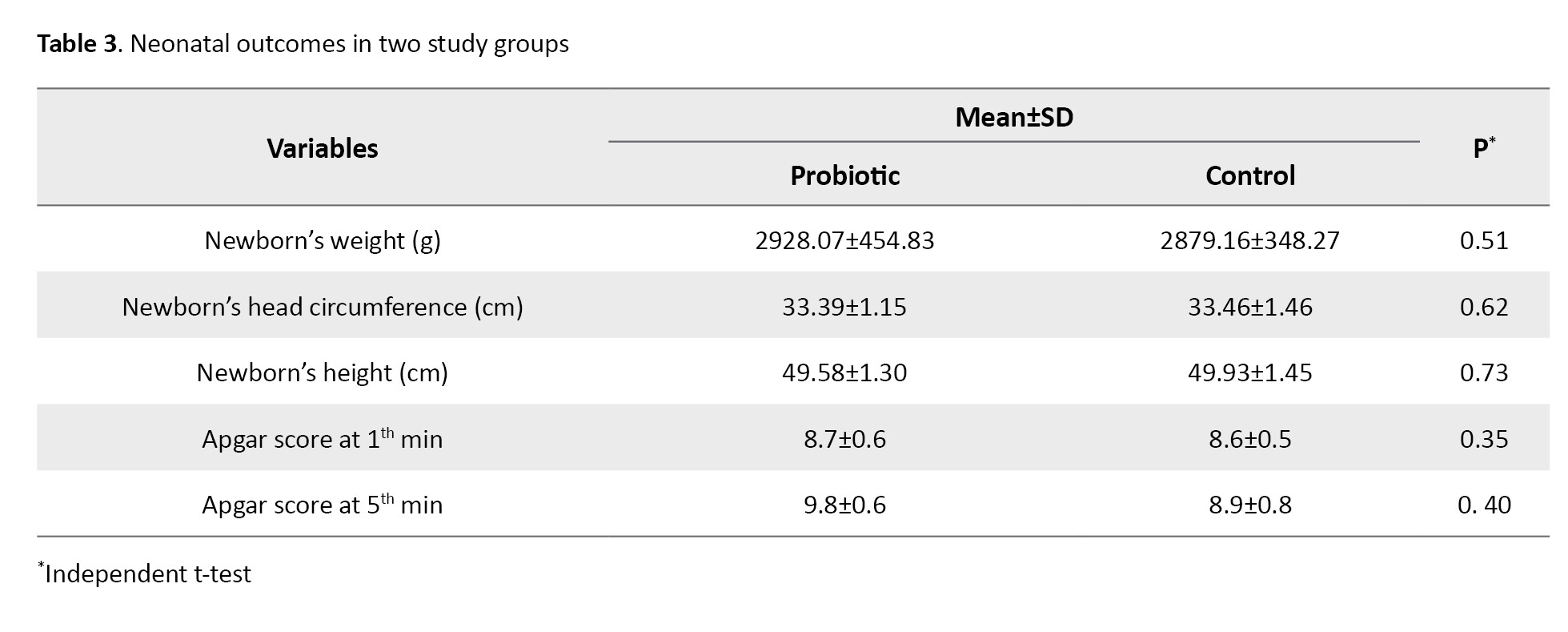
Neonatal characteristics of the two groups are presented in Table 3. Results showed that in the probiotic and control groups, the newborn’s weight was 2928.07±454.83 and 2879.16±348.27; head circumstance was 33.39±1.15 and 33.46±1.46; height was 49.58±1.30 and 49.93±1.45; Apgar score at 1 minute was 8.7±0.6 and 8.6±0.5, and Apgar score at 5 minutes was 9.8±0.6 and 8.9±0.8 after birth, respectively. The results showed no significant differences in these variables between the two groups. Both groups were in the optimal range of Apgar score. No side effects were reported in any group.
Discussion
The results of this clinical trial showed that the efficacy of adjuvant administration of probiotics was not significantly different in preventing PTB in pregnant women at high risk for PTB than the 17α-OHP IM injection alone. Other pregnancy outcomes, including PPROM, mode of delivery, newborn weight, height, head circumference, and Apgar score at 1 and 5 minutes after birth, were not significantly different, either.
Probiotics have been reported as a preventive strategy for PTB [16]. The present study challenges previous studies on the effectiveness of starting using probiotics in the 16th week of pregnancy in preventing PTB and other maternal and neonatal outcomes. A previous study showed that probiotics containing lactobacilli were effective in treating bacterial vaginosis [17] and although it has not been established, the prevention of PTB, if present, is negative [18], because there is a connection between the use of probiotics and treatment of bacterial vaginosis which has a potential role in preventing PTB [19]. In a review study on the effects of prenatal probiotics on preventing PTB [10], probiotics were found to reduce the risk of genital tract infections (bacterial vaginosis) by 81%. However, there was insufficient evidence to determine whether probiotics reduced the incidence of PTB. No side effects after using the probiotics or prebiotics during pregnancy have been reported. However, a meta-analysis by Dugoua et al. [20] showed no differences in gestational age in the probiotic compared to the non-probiotic group.
Previous studies have shown no correlation between the label and the actual content of probiotic products in many cases [21, 22] and the main properties of some probiotic strains can be affected by industrial production processes, which can lead to their instability. Therefore, our results cannot be related to the commercial probiotic products. Based on prospective cohort studies, the use of fermented dairy products can be considered a valuable nutritional intervention for all pregnant women [23-25]. For example, consuming approximately three ounces of a fermented dairy product per day was associated with a significant reduction in the risk of spontaneous PTB [8]. The populations of these studies probably differ from the pregnant women in the present study in terms of ethnicity, genetics and immune system, since microbiota colonization varies by race/ethnicity and geographic location [26-30]. The women in our study were Iranian. The present study was designed to be clinically applicable in terms of gestational age at initiation of probiotic administration, as well as dosage and type of probiotics. Therefore, although our study failed to provide beneficial effects for probiotic supplementation during pregnancy compared to placebo, other microbial species or dosages may be effective. It is also possible that starting the intervention before pregnancy or continuing it for a longer period of time could affect the results. The consumption of probiotics in the present study was started in the 16th gestational week.
It is recommended that the protective effect of fermented dairy products, as well as the effect of their absence as a risk factor for PTB, be investigated to improve understanding of health outcomes during pregnancy and facilitate the implementation of effective health promotion strategies.
The strengths of this study included the use of rigorous and extensive inclusion and exclusion criteria, a large sample size with different dietary habits, and a wide range of probiotic product intake doses. However, there were some limitations/disadvantages. We did not assess women’s adherence to the assigned intervention using stool analysis, as greater adherence may be associated with better outcomes. Also, women’s dietary intake before and during pregnancy was not assessed.
Based on the results, it seems that the use of probiotics as adjuvants from the 16th to the 37th gestational week, along with 17α-OHP IM injection, does not reduce the risk of spontaneous PTB or improve other neonatal and maternal outcomes in pregnant women at high risk for PTB. However, further randomized clinical trials are needed to investigate the use of different species and doses of probiotics for a longer period to improve our understanding of the role of gut microbiota in pregnancy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran (Code: IR.YUMS.REC.1399.132) and was registered by the Iranian Registry of Clinical Trials (Code: IRCT20201108049300N1). Written informed consent was obtained from all patients. All procedures involving human participants performed in this study were in accordance with the ethical standards of the Helsinki Declaration and its later amendments.
Funding
This research was financially supported by Yasuj University of Medical Sciences, Yasuj, Iran (Grant No.: 980077).
Authors' contributions
Data collection: Raziyeh Vanda, Mansoureh Moghadam-Sangcholi and Hossein Sadeghi; Data analysis: Fatemeh Bazarganipour; Draft preparation: Fatemeh Bazarganipour, Marcello Iriti and Seyed-Abdolvahab Taghavi; Supervision: Raziyeh Vanda and Fatemeh Bazarganipour; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research of Yasuj University of Medical Sciences, Yasuj, Iran, for the financial support, and all participants for their cooperation in this study.
References
Preterm Birth (PTB) is defined as birth before 37 weeks of gestation [1]. Up to 50% of PTBs are associated with maternal infection [2]. Genitourinary infections, including urinary tract infections, bacterial vaginosis and yeast vaginitis, annually affect about one billion women in the world. In recent years, genitourinary infections have been an important risk factor for PTB [3]. The most common route for urogenital pathogens that cause preterm labor is the ascending pathway [4]. Proteolytic enzymes act directly on the collagen of the cervix and fetal membranes and lead to early thinning of the cervix and its insufficiency, weakening of the fetal membranes, and subsequently Preterm premature rupture of membranes (PPROM) [5].
Probiotics, as living microorganisms, can provide health benefits to the host when administered in sufficient amounts. Probiotics displace and kill pathogens, and modulate the immune response by interfering with the inflammatory cascades that lead to PTB [6]. The mechanism of action of probiotics in the vagina is probably multifactorial. The production of lactic acid, bacteriocins, and hydrogen peroxide and the immune response modulation can be the possible mechanisms [7].
A cohort study in Norway reported a significant protective effect against spontaneous PTB in women who had a high intake of probiotic milk [8]. A review study comparing probiotics with placebo reported no statistically significant difference in gestational age at birth [9]. Another review study reported no significant finding that probiotics increased or decreased the incidence of PTB [10]. Regarding the effect of probiotic oral supplementation in pregnancy on the risk of PTB, no benefit or harm has been reported, and more studies are needed in this field. However, it has been proposed that the combination of L. acidophilus and Bifidobacterium bifidum probiotic species may be more useful in improving pregnancy outcomes [11]. Progesterone has been shown to suppress the contractions of the myometrium; therefore, one strategy is the use of supplemental progestogens, including Intramuscular (IM) injection of 17α-hydroxyprogesterone caproate (17α-OHP) in women with a singleton pregnancy and a history of singleton spontaneous PTB [12]. The 17α-OHP has been recommended to prevent PTB by the American College of Obstetricians and Gynecologists (ACOG) and the society for maternal-fetal medicine (SMFM). In August 2021, ACOG recommended that women with a singleton pregnancy and a history of spontaneous PTB should receive progesterone supplementation vaginally or by IM injection [13]. However, considering the consequences of PTB and the lack of theoretical agreement on the use of different drugs for the prevention of PTB, it is appropriate to conduct further studies in this field. Due to the existing contradictions regarding the effects of probiotics in women at high risk of PTB and considering the benefits of probiotics (bacteriotherapy and immune regulation), this study aims to investigate the efficacy of adjuvant administration of probiotics on the spontaneous PTB and the related pregnancy outcomes in pregnant women at high risk for PTB receiving 17α-OHP.
Materials and Methods
This is a randomized clinical trial in compliance with the CONSORT guidelines [14]. The study population consists of all pregnant women referred to the gynecology clinics in Yasuj, Iran, from 2020 to 2022 who were receiving 17α-OHP injection (250 mg, intramuscular injection [IM]). Using the related formula, and by considering α=0.05, β=0.80, a 15% sample dropout rate, 20% cesarean rate in the probiotic group, and 46.7% cesarean rate in the control group according to the findings of Badehnoosh et al. [15], the sample size was determined to be 60 per group. First, 132 participants were assessed for eligibility, of whom 12 were excluded due to not meeting inclusion criteria and declined to participate. Finally, 120 patients were included. Inclusion criteria were a history of high-risk PTB (including a history of PTB or pregnancy termination at the second trimester), willingness to participate in the study, age 18-45 years, gestational age 16-24 weeks, no syphilis, gonorrhea or HIV, no elective or emergency cervical cerclage, and no maternal insulin-dependent diabetes mellitus, hypertension, lupus and clinical chorioamnionitis based on medical records. Exclusion criteria were unwillingness to continue participation in the study, failure to complete the treatment, taking drugs that affect the intestinal microbial flora (such as antibiotics), occurrence of any genital or urinary tract infection that required antibiotic treatment during the trial, having a fetus with congenital malformations and abnormal scan anomalies, or clinical chorioamnionitis.
Eligible participants were assigned to two groups: Group A: Lactofem capsule (containing Lactobacillus acidophilus 2×109 cfu/g, Bifidobacterium bifidus 2×109 cfu/g, Lactobacillus rutri 2×109 cfu/g, Lactobacillus fermentum 2×109 cfu/g; bio-capsule weight of 500 mg made by Zist Takhmir Company, Iran, administered orally and daily from the 16th to the 37th week of pregnancy) and Group B: Placebo capsules containing starch powder (not harmful during pregnancy) prepared by the medicinal plants laboratory, Yasuj University of Medical Sciences. Capsules in two groups were similar in shape and package. The randomization of patients was performed with a random allocation software using the block randomization method. To hide the treatment options, the list of treatments was placed in sealed and numbered envelopes (in a sequencing order). Participants and physicians were blinded to the allocation and were not aware of group allocation.
Socio-demographic and reproductive information including age, body mass index (BMI), educational level, occupation (housewife or employed), gravidity, history of abortion and parity were first recorded. All participants were informed that they could leave the study at any time. All participants followed the prenatal care and pregnancy outcome in Shahid Mofatteh gynecology clinic under the supervision of one gynecologist. Obstetric information included the history of PPROM, preterm labor (late and early) and mode of delivery (Instrumental, cesarean section, or normal vaginal delivery [NVD]). Neonatal information included weight, height and head circumference of the newborn and the Apgar score (1 and 5 minutes after birth). The side effects including fever, itching, diarrhea, vomiting, or other gastrointestinal symptoms were also recorded. PTB was considered as the primary outcome and pregnancy-related complications as the secondary outcome (Figure 1).

Data analysis was carried out in SPSS software, version 21 using descriptive statistics (frequency, percent, Mean±SD), chi-square test and independent t-test. Kolmogorov–Smirnov test was used to examine the normality of data distribution. The significance level was set at 0.05. There were no missing data. Therefore, no missing imputation technique was used.
Results
Two women from the probiotic group left the study because they were unwilling to continue the treatment. Therefore, the data of 118 women was analyzed. Their characteristics are presented in Table 1.

There were no significant differences between the two groups in terms of age, BMI, educational level, occupation, gravidity, abortion, or parity. Table 2 presents the obstetric information for the two groups. As can be seen, 8.62% and 15% of women in the probiotic and placebo groups had PTB before the 34th week of pregnancy, while 12.06% and 16.7% had PTB from the 34th to the 37th week of pregnancy, respectively. Results also showed no significant differences between the two groups regarding PPROM or mode of delivery.

Neonatal characteristics of the two groups are presented in Table 3. Results showed that in the probiotic and control groups, the newborn’s weight was 2928.07±454.83 and 2879.16±348.27; head circumstance was 33.39±1.15 and 33.46±1.46; height was 49.58±1.30 and 49.93±1.45; Apgar score at 1 minute was 8.7±0.6 and 8.6±0.5, and Apgar score at 5 minutes was 9.8±0.6 and 8.9±0.8 after birth, respectively. The results showed no significant differences in these variables between the two groups. Both groups were in the optimal range of Apgar score. No side effects were reported in any group.

Discussion
The results of this clinical trial showed that the efficacy of adjuvant administration of probiotics was not significantly different in preventing PTB in pregnant women at high risk for PTB than the 17α-OHP IM injection alone. Other pregnancy outcomes, including PPROM, mode of delivery, newborn weight, height, head circumference, and Apgar score at 1 and 5 minutes after birth, were not significantly different, either.
Probiotics have been reported as a preventive strategy for PTB [16]. The present study challenges previous studies on the effectiveness of starting using probiotics in the 16th week of pregnancy in preventing PTB and other maternal and neonatal outcomes. A previous study showed that probiotics containing lactobacilli were effective in treating bacterial vaginosis [17] and although it has not been established, the prevention of PTB, if present, is negative [18], because there is a connection between the use of probiotics and treatment of bacterial vaginosis which has a potential role in preventing PTB [19]. In a review study on the effects of prenatal probiotics on preventing PTB [10], probiotics were found to reduce the risk of genital tract infections (bacterial vaginosis) by 81%. However, there was insufficient evidence to determine whether probiotics reduced the incidence of PTB. No side effects after using the probiotics or prebiotics during pregnancy have been reported. However, a meta-analysis by Dugoua et al. [20] showed no differences in gestational age in the probiotic compared to the non-probiotic group.
Previous studies have shown no correlation between the label and the actual content of probiotic products in many cases [21, 22] and the main properties of some probiotic strains can be affected by industrial production processes, which can lead to their instability. Therefore, our results cannot be related to the commercial probiotic products. Based on prospective cohort studies, the use of fermented dairy products can be considered a valuable nutritional intervention for all pregnant women [23-25]. For example, consuming approximately three ounces of a fermented dairy product per day was associated with a significant reduction in the risk of spontaneous PTB [8]. The populations of these studies probably differ from the pregnant women in the present study in terms of ethnicity, genetics and immune system, since microbiota colonization varies by race/ethnicity and geographic location [26-30]. The women in our study were Iranian. The present study was designed to be clinically applicable in terms of gestational age at initiation of probiotic administration, as well as dosage and type of probiotics. Therefore, although our study failed to provide beneficial effects for probiotic supplementation during pregnancy compared to placebo, other microbial species or dosages may be effective. It is also possible that starting the intervention before pregnancy or continuing it for a longer period of time could affect the results. The consumption of probiotics in the present study was started in the 16th gestational week.
It is recommended that the protective effect of fermented dairy products, as well as the effect of their absence as a risk factor for PTB, be investigated to improve understanding of health outcomes during pregnancy and facilitate the implementation of effective health promotion strategies.
The strengths of this study included the use of rigorous and extensive inclusion and exclusion criteria, a large sample size with different dietary habits, and a wide range of probiotic product intake doses. However, there were some limitations/disadvantages. We did not assess women’s adherence to the assigned intervention using stool analysis, as greater adherence may be associated with better outcomes. Also, women’s dietary intake before and during pregnancy was not assessed.
Based on the results, it seems that the use of probiotics as adjuvants from the 16th to the 37th gestational week, along with 17α-OHP IM injection, does not reduce the risk of spontaneous PTB or improve other neonatal and maternal outcomes in pregnant women at high risk for PTB. However, further randomized clinical trials are needed to investigate the use of different species and doses of probiotics for a longer period to improve our understanding of the role of gut microbiota in pregnancy.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran (Code: IR.YUMS.REC.1399.132) and was registered by the Iranian Registry of Clinical Trials (Code: IRCT20201108049300N1). Written informed consent was obtained from all patients. All procedures involving human participants performed in this study were in accordance with the ethical standards of the Helsinki Declaration and its later amendments.
Funding
This research was financially supported by Yasuj University of Medical Sciences, Yasuj, Iran (Grant No.: 980077).
Authors' contributions
Data collection: Raziyeh Vanda, Mansoureh Moghadam-Sangcholi and Hossein Sadeghi; Data analysis: Fatemeh Bazarganipour; Draft preparation: Fatemeh Bazarganipour, Marcello Iriti and Seyed-Abdolvahab Taghavi; Supervision: Raziyeh Vanda and Fatemeh Bazarganipour; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research of Yasuj University of Medical Sciences, Yasuj, Iran, for the financial support, and all participants for their cooperation in this study.
References
- Quinn JA, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016; 34(49):6047-56. [DOI:10.1016/j.vaccine.2016.03.045] [PMID]
- Dahman HAB. Risk factors associated with preterm birth: A retrospective study in Mukalla Maternity and Childhood Hospital, Hadhramout Coast/Yemen. Sudan J Paediatr. 2020; 20(2):99-110.[DOI:10.24911/SJP.106-1575722503] [PMID]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007; 25(1):21-39. [DOI:10.1055/s-2006-956773] [PMID]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000; 342(20):1500-7. [DOI:10.1056/NEJM200005183422007] [PMID]
- McGregor JA, French JI. Pathogenesis to treatment: Preventing preterm birth mediated by infection. Infect Dis Obstet Gynecol. 1997; 5(2):106-14. [DOI:10.1155/S1064744997000173] [PMID]
- Reid G. The development of probiotics for women's health. Can J Microbiol. 2017; 63(4):269-277. [DOI:10.1139/cjm-2016-0733] [PMID]
- Reid G, Burton J, Devillard E. The rationale for probiotics in female urogenital healthcare. MedGenMed. 2004; 6(1):49. [PMID]
- Myhre R, Brantsæter AL, Myking S, Gjessing HK, Sengpiel V, Meltzer HM, et al. Intake of probiotic food and risk of spontaneous preterm delivery. Am J Clin Nutr. 2011; 93(1):151-7. [DOI:10.3945/ajcn.110.004085] [PMID]
- Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational Diabetes. Cochrane Database Syst Rev. 2021; 4(4):CD009951. [DOI:10.1002/14651858] [PMID]
- Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev. 2007; 2007(1):CD005941. [DOI:10.1002/14651858.CD005941]
- Grev J, Berg M, Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2018; 12(12):CD012519. [DOI:10.1002/14651858.CD012519]
- Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008; 198(6):633.e1-8. [DOI:10.1016/j.ajog.2007.11.047] [PMID]
- Society for Maternal-Fetal Medicine (SMFM); SMFM Publications Committee. Society for Maternal-Fetal Medicine Statement: Response to the Food and Drug Administration's withdrawal of 17-alpha hydroxyprogesterone caproate. Am J Obstet Gynecol. 2023; 229(1):B2-6.[DOI:10.1016/j.ajog.2023.04.012] [PMID]
- Junqueira DR, Zorzela L, Golder S, Loke Y, Gagnier JJ, Julious SA, et al. CONSORT Harms 2022 statement, explanation, and elaboration: Updated guideline for the reporting of harms in randomised trials. BMJ. 2023; 381:e073725. [DOI:10.1136/bmj-2022-073725] [PMID]
- Badehnoosh B, Karamali M, Zarrati M, Jamilian M, Bahmani F, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J Matern Fetal Neonatal Med. 2018; 31(9):1128-36. [DOI:10.1080/14767058.2017.1310193] [PMID]
- van Zijl MD, Koullali B, Mol BW, Pajkrt E, Oudijk MA. Prevention of preterm delivery: Current challenges and future prospects. Int J Womens Health. 2016; 8:633-45. [DOI:10.2147/IJWH.S89317] [PMID]
- Chen R, Li R, Qing W, Zhang Y, Zhou Z, Hou Y, et al. Probiotics are a good choice for the treatment of bacterial vaginosis: A meta-analysis of randomized controlled trial. Reprod Health. 2022; 19(1):137. [DOI:10.1186/s12978-022-01449-z] [PMID]
- Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009; (4):CD006289. [DOI:10.1002/14651858.CD006289]
- US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US preventive services task force recommendation statement. JAMA. 2020; 323(13):1286-92. [DOI:10.1001/jama.2020.2684] [PMID]
- Dugoua JJ, Machado M, Zhu X, Chen X, Koren G, Einarson TR. Probiotic safety in pregnancy: A systematic review and meta-analysis of randomized controlled trials of Lactobacillus, Bifidobacterium, and Saccharomyces spp. J Obstet Gynaecol Can. 2009; 31(6):542-52. [DOI:10.1016/S1701-2163(16)34218-9] [PMID]
- Zawistowska-Rojek A, Zareba T, Mrówka A, Tyski S. Assessment of the microbiological status of probiotic products. Pol J Microbiol. 2016; 65(1):97-104. [DOI:10.5604/17331331]
- Drago L, Rodighiero V, Celeste T, Rovetto L, De Vecchi E. Microbiological evaluation of commercial probiotic products available in the USA in 2009. J Chemother. 2010; 22(6):373-7. [DOI:10.1179/joc.2010.22.6.373] [PMID]
- Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, et al. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. 2016; 133(17):1645-54. [DOI:10.1161/CIRCULATIONAHA.115.018410] [PMID]
- Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 Diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018; 15(10):e1002670. [DOI:10.1371/journal.pmed.1002670] [PMID]
- Heppe DH, van Dam RM, Willemsen SP, den Breeijen H, Raat H, Hofman A, et al. Maternal milk consumption, fetal growth, and the risks of neonatal complications: The Generation R Study. Am J Clin Nutr. 2011; 94(2):501-9. [DOI:10.3945/ajcn.111.013854] [PMID]
- Amato KR, Arrieta MC, Azad MB, Bailey MT, Broussard JL, Bruggeling CE, et al. The human gut microbiome and health inequities. Proc Natl Acad Sci USA. 2021; 118(25):e2017947118. [DOI:10.1073/pnas.2017947118] [PMID]
- Bowyer RCE, Jackson MA, Le Roy CI, Ni Lochlainn M, Spector TD, Dowd JB, et al. Socioeconomic status and the gut microbiome: A TwinsUK cohort study. Microorganisms. 2019; 7(1):17. [DOI:10.3390/microorganisms7010017] [PMID]
- Harrison CA, Taren D. How poverty affects diet to shape the microbiota and chronic disease. Nat Rev Immunol. 2018; 18(4):279-287. [DOI:10.1038/nri.2017.121] [PMID]
- Lewis CR, Bonham KS, McCann SH, Volpe AR, D'Sa V, Naymik M, et al. Family SES is associated with the gut microbiome in infants and children. Microorganisms. 2021; 9(8):1608. [DOI:10.3390/microorganisms9081608] [PMID]
- Miller GE, Engen PA, Gillevet PM, Shaikh M, Sikaroodi M, Forsyth CB, et al. Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PLoS One. 2016; 11(2):e0148952. [DOI:10.1371/journal.pone.0148952] [PMID]
Article Type : Research |
Subject:
Special
Received: 2024/06/16 | Accepted: 2025/01/19 | Published: 2025/04/1
Received: 2024/06/16 | Accepted: 2025/01/19 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



