Mon, Feb 2, 2026
Volume 34, Issue 3 (6-2024)
JHNM 2024, 34(3): 211-220 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jangpour M, Jesmi A A, Kooshki A, Mahdavifar N, Taj A. Effect of Synbiotic Supplementation on Fatigue and Sleep Quality in End-stage Renal Disease Patients: A Randomized Clinical Trial. JHNM 2024; 34 (3) :211-220
URL: http://hnmj.gums.ac.ir/article-1-2369-en.html
URL: http://hnmj.gums.ac.ir/article-1-2369-en.html
1- MSc Student of Critical Care Nursing, School of Nursing, Student Research Committee, Sabzevar University of Medical Sciences, Sabzevar, Iran.
2- Assistant Professor, School of Nursing, Iranian Research Centre on Healthy Aging, Sabzevar University of Medical Sciences, Sabzevar, Iran. ,jesmiaa@gmail.com
3- Professor, Department of Biochemistry and Nutrition, School of Medicine, Iranian Research Center on Healthy Aging, Sabzevar University of Medical Sciences, Sabzevar, Iran.
4- MSc in Epidemiology, Department of Epidemiology and Biostatistics, School of Medicine, Sabzevar University of Medical Sciences, Sabzevar, Iran.
5- Assistant Professor, School of Paramedicine, Non-Communicable Diseases Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran.
2- Assistant Professor, School of Nursing, Iranian Research Centre on Healthy Aging, Sabzevar University of Medical Sciences, Sabzevar, Iran. ,
3- Professor, Department of Biochemistry and Nutrition, School of Medicine, Iranian Research Center on Healthy Aging, Sabzevar University of Medical Sciences, Sabzevar, Iran.
4- MSc in Epidemiology, Department of Epidemiology and Biostatistics, School of Medicine, Sabzevar University of Medical Sciences, Sabzevar, Iran.
5- Assistant Professor, School of Paramedicine, Non-Communicable Diseases Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran.
Full-Text [PDF 574 kb]
(480 Downloads)
| Abstract (HTML) (1297 Views)
Full-Text: (788 Views)
Introduction
Chronic kidney disease is one of the public health issues in the world. End-stage renal disease (ESRD) occurs when kidney function declines less than 10% to 15% of its normal level [1]. The prevalence of chronic kidney disease is estimated to be 8% to 16% worldwide [2]. In most developed countries, hemodialysis is the standard strategy for treatment at the beginning of ESRD and in patients over 65 years [3].
Fatigue is one of the most common complications experienced by hemodialysis patients. At least half of the patients complain of persistent fatigue, and about 86% report fatigue after dialysis [3, 4]. Also, about 50% to 83% of these patients suffer from poor sleep quality [5]. The onset of chronic kidney disease and hemodialysis, followed by complications such as fatigue and decreased sleep quality, significantly affect the patients’ quality of life (QoL) [6]. In a study conducted by Bossola et al. on fatigue in patients undergoing hemodialysis, fatigue unrelated to depression alone can increase the risk of mortality in these patients [7]. Factors such as anemia, inflammation, and hemodialysis may trigger the onset of the fatigue cycle and then the patients’ cognitive, behavioral, and emotional responses to early symptoms such as negative beliefs, depression, anxiety, and maladaptive behaviors. Gradually, this cycle leads to physiological complications such as systemic inflammation, central nervous system and endocrine disorders, and poor sleep quality. Finally, the fatigue cycle can lead to poor clinical outcomes in the patient [3].
Recently, the role of inflammatory factors as a primary agent in the fatigue cycle has become more prominent [8]. Therefore, it has been suggested that reducing systemic inflammation can effectively reduce fatigue, improve sleep quality, and ultimately improve the QoL [9, 10].
Scheper et al. introduced the intestine as a neglected organ in chronic kidney disease and its associated uremia [11]. Recent studies have shown that imbalances and quantitative and qualitative changes in the composition and activity of intestinal microbiota in ESRD patients accelerate the disease’s progression, uremia, and its complications, such as fatigue and poor sleep quality [12, 13]. High levels of potassium and phosphate in patients undergoing hemodialysis cause them to avoid the source of these elements. Consequently, patients are deprived of a rich source of indigestible complex carbohydrates, which are the main nutrients for intestinal bacteria [14]. The brain-intestine-kidney axis plays an essential role in the body’s normal homeostasis [15].
Probiotics are defined as beneficial living microorganisms if taken at an optimum level. Prebiotics are also described as dietary supplements (such as inulin) that support the growth of probiotics. Synbiotics are a combination of probiotics and prebiotics that may have a more significant effect on gastrointestinal health and systemic inflammation in hemodialysis patients due to their synergistic effect [9-14].
Therefore, it is hypothesized that changes in intestinal microbiota following synbiotic supplements can improve metabolic health by reducing inflammatory factors. Kooshki et al. reported that taking 8 weeks of synbiotic dietary supplement in hemodialysis patients can reduce inflammatory factors (C-reactive protein), malondialdehyde, total cholesterol, and low-density lipoprotein [16]. Also, considering the important role of anemia in chronic fatigue caused by chronic diseases, a systematic review and meta-analysis showed increasing iron absorption with probiotics consumption [17].
Considering that several studies have investigated the effect of synbiotic supplementation on fatigue, inflammatory and anemic factors, and improving the immune system of patients [18, 19], we evaluated the effects of synbiotic supplement therapy on improving sleep quality and fatigue in ESRD patients undergoing hemodialysis.
Materials and Methods
The study was a double-blind, randomized, placebo-controlled clinical trial. The required sample size in this project was based on the Srinivasa et al. study [9]. Considering the Mean±SD of fatigue scores before and after using synbiotic supplementation were reported as 42.76±22.22 and 47.50±21.87, respectively, α=0.05, and β=20% for each group, 26 people were obtained for each study group. This number increased to 30 people for each group, considering the possibility of 20% drop-out.
The 60 clinically stable hemodialysis (HD) patients aged 18 to 65 years receiving HD thrice weekly at the Dialysis Department of the Educational Center in Quchan, Iran, for at least 3 months before the onset of the study were enrolled. Dialysis duration was 3 to 4.5 hours per session, three times per week, with a blood flow of 250 mL/min and a dialysate flow of 500 mL/min.
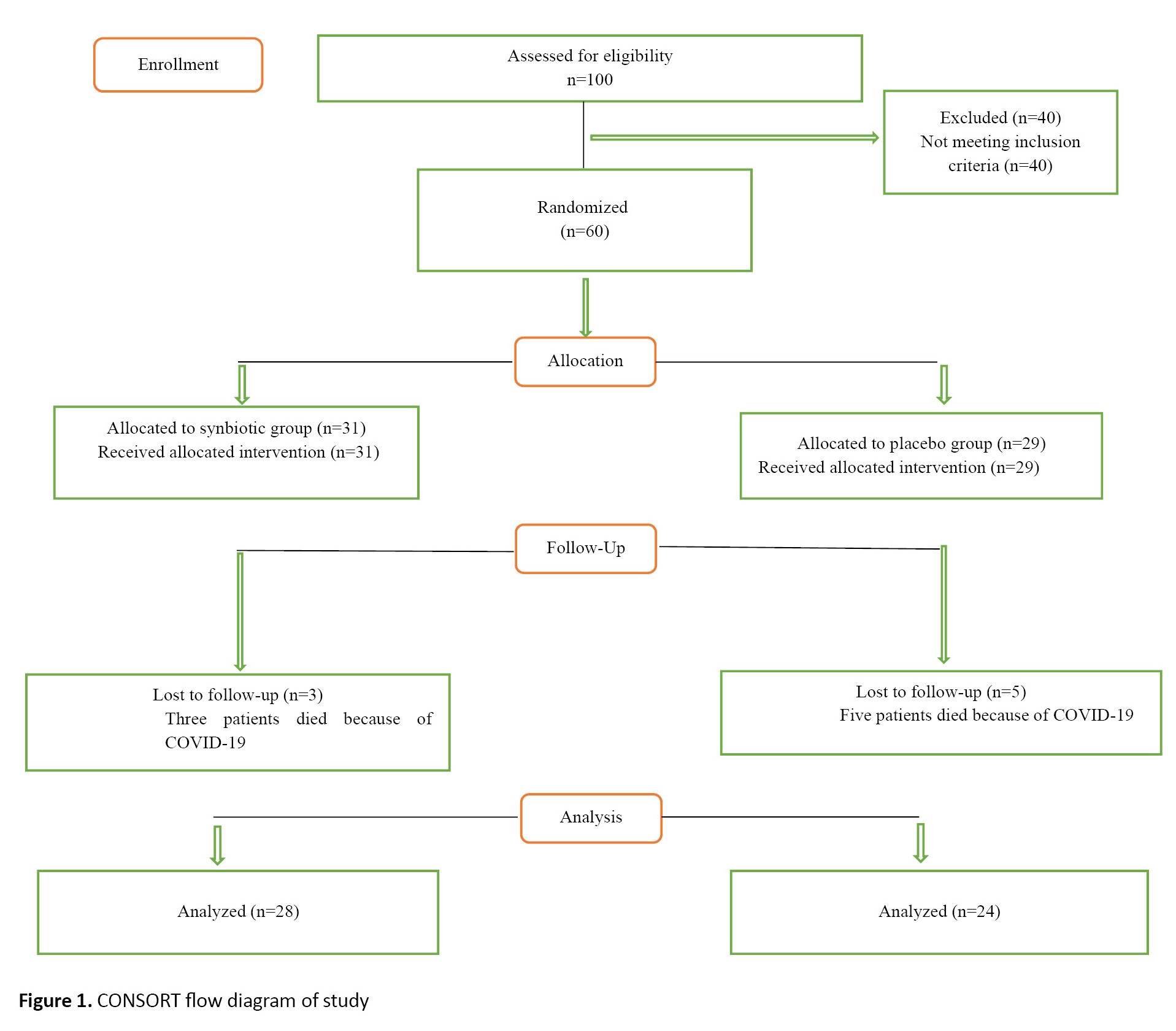
The non-entry criteria based on the patient’s medical file included patients who had undergone kidney transplants or were likely to receive a transplant, those medically diagnosed with severe infections, diabetes, malignancy, chronic liver disease (hepatitis B, hepatitis C), gastrointestinal diseases (inflammatory bowel disease, ulcerative colitis, Crohn disease, celiac disease, lactose intolerance or allergy, irritable bowel syndrome), diabetic foot infection, pregnant or lactating mothers, and those with hemoglobin less than 8 mg/dL. According to the patient’s report, the Pittsburgh sleep quality index (PSQI) score is less than 6 (good sleep quality), the Chalder fatigue scale (CFS) score is less than 18, receive antioxidant vitamin supplements, prebiotics, probiotics, synbiotics, or antibiotics within one month of study (Figure 1). Demographic, CFS and PSQI questionnaires reported and measured patients’ characteristics, as well as fatigue and sleep quality. The CFS was first published by Chalder et al. This questionnaire contains 11 questions and two general domains for examining physical and emotional fatigue during the last month. Each question is assigned a score between 0 and 3; the total result is between 0 and 33. The global score also spans physical fatigue (measured by items 1–7) and psychological fatigue (measured by items 8–11). If the patient scores above 18, he or she will be considered tired; the patient suffers from more fatigue with a higher score [20]. In Iran, for the first time, Nasri et al. evaluated the validity of this questionnaire [21]. The fatigue tool’s validity was assessed using the content validity method, and its reliability was assessed using the internal validity method. The Cronbach α coefficient was calculated to be 0.79.
The PSQI is a self-report questionnaire designed by Buysse et al. and assesses sleep quality over the past month. The questionnaire includes 19 questions about 7 important components of sleep (mental quality of sleep, duration of sleep, duration of sleep, sleep efficiency, sleep disorders, use of sleeping pills, and inefficiency latency of sleep). Each component is assigned a score between 0 and 3, so the overall PSQI score is between 0 and 21. A score between 0 and 5 means good sleep quality, while a score between 6 and 21 means poor sleep quality [22]. This study used the psychometric Persian version of this questionnaire [23].
This research was a double-blind, randomized placebo study. The sampling method was convenient, and permuted block randomization was used to randomize the data. In this regard, 15 blocks with the size of four were formed (A and B for the intervention groups, C and D for the placebo group). Neither the patients, nephrologists, nor the researcher performing patients’ evaluation were aware of the group assignment. The baseline clinical evaluation included an anthropometric examination. Also, the PSQI and CFS questionnaires were administered to all patients. During the intervention, patients were asked to maintain a steady diet, engage in physical activity, take probiotic drugs, and not take supplements other than those provided by the trial.
The intervention group took two synbiotic capsules daily (one after lunch and the other after dinner), and the control group took two placebo capsules (containing 500 mg corn starch) daily at the same time for 8 weeks. These drugs are safe and have been used in similar studies [24-26]. Synbiotic and placebo capsules are similar in appearance and are marked only with the label “A” or “B” on the box. Boxes are tagged by a third party not directly involved in this study. Each Lactocore synbiotic capsule (500 mg) (produced by Zist Takhmir Company of Iran) contains high amounts of 12 beneficial and safe bacterial strains and fructooligosaccharide as a prebiotic. The strains used in this product include Lactobacillus rhamnosus, Lactobacillus Helveticus, Lactobacillus casei, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium breve, Lactobacillus bulgaricus, Bifidobacterium Langum, Lactobacillus plantarum, Bifidobacterium bifidum, Lactobacillus garii, and Streptococcus thermophilus. The placebo powder was comparable in color, texture, and taste to the synbiotics and prebiotics but contained only corn starch. Each box contained 30 capsules. The participants were given four boxes for the entire 2-month intervention (two boxes at the beginning of each month). In addition, the patients continued to take their daily medications during the study.
In subsequent visits, the patients were followed up by phone twice weekly to comply with the intervention. They were asked to take one capsule after lunch and one after dinner, which improved their adherence to the intervention. In addition, the samples were asked to record their daily intake of supplements or placebo in the notebook provided and to return any remaining capsules in the boxes at the next visit. At the end of 8 weeks, if the number of remaining capsules for each participant was more than 10% of the prescribed capsules, that participant was removed from the intervention.
Statistical analysis was performed using SPSS software, version 21 (Chicago, IL). Quantitative variables were described as Mean±SD, and qualitative variables as number (percentage). The repeated measures test was used because the outcome variable was measured three times in each group. Demographic variables were analyzed using the chi-square test, Fisher exact test, or independent sample t-test, as appropriate. Also, sphericity assumed Greenhouse-Geisser was used to check the homogeneity of the data. For all analyses, P<0.05 were considered statistically significant.
Results
Of 100 patients referred for hemodialysis treatment, 60 were included in the study and assigned to the synbiotic group (31 patients) and the placebo group (29 patients).
The data of 3 participants from the synbiotic group and 5 participants from the placebo group were excluded due to death after catching COVID-19 during the follow-up period. The remaining samples (n=52, 26 males and 26 females) were randomly allocated to the placebo (n=24, 12 males and 12 females) or the synbiotic supplement (n=28, 14 males and 14 females) groups.
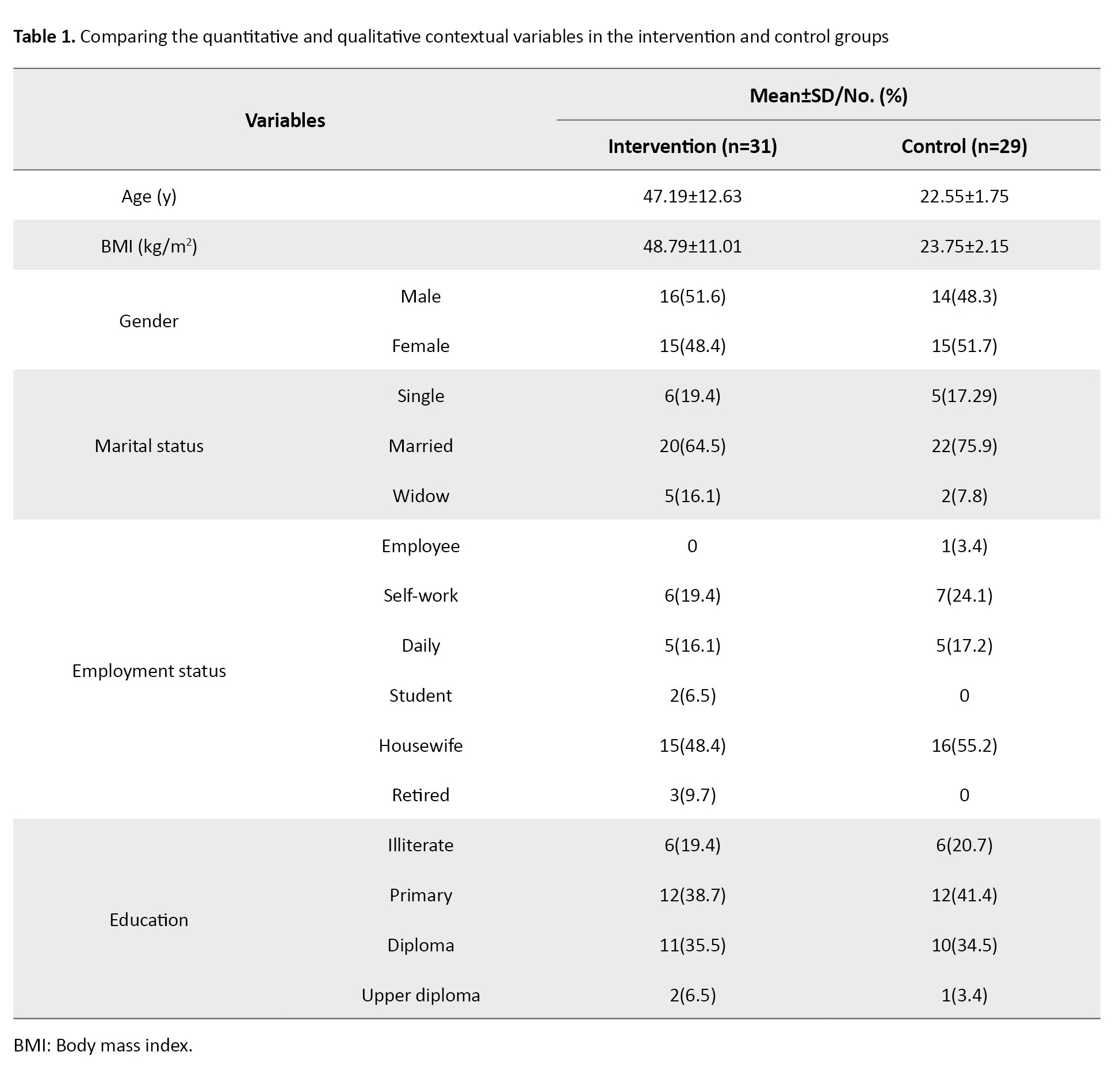
Their mean age in the synbiotic group was 47.19±12.63 years, and in the placebo group, 48.79±11.01 years. All enrolled patients received erythropoietin and intravenous iron as a regular treatment following our regional HD guidelines (Table 1).
There were no significant side effects associated with the study treatments. However, some adverse events assessed as unrelated to study participation occurred among two patients in the placebo group: Oral mucositis and respiratory problems.
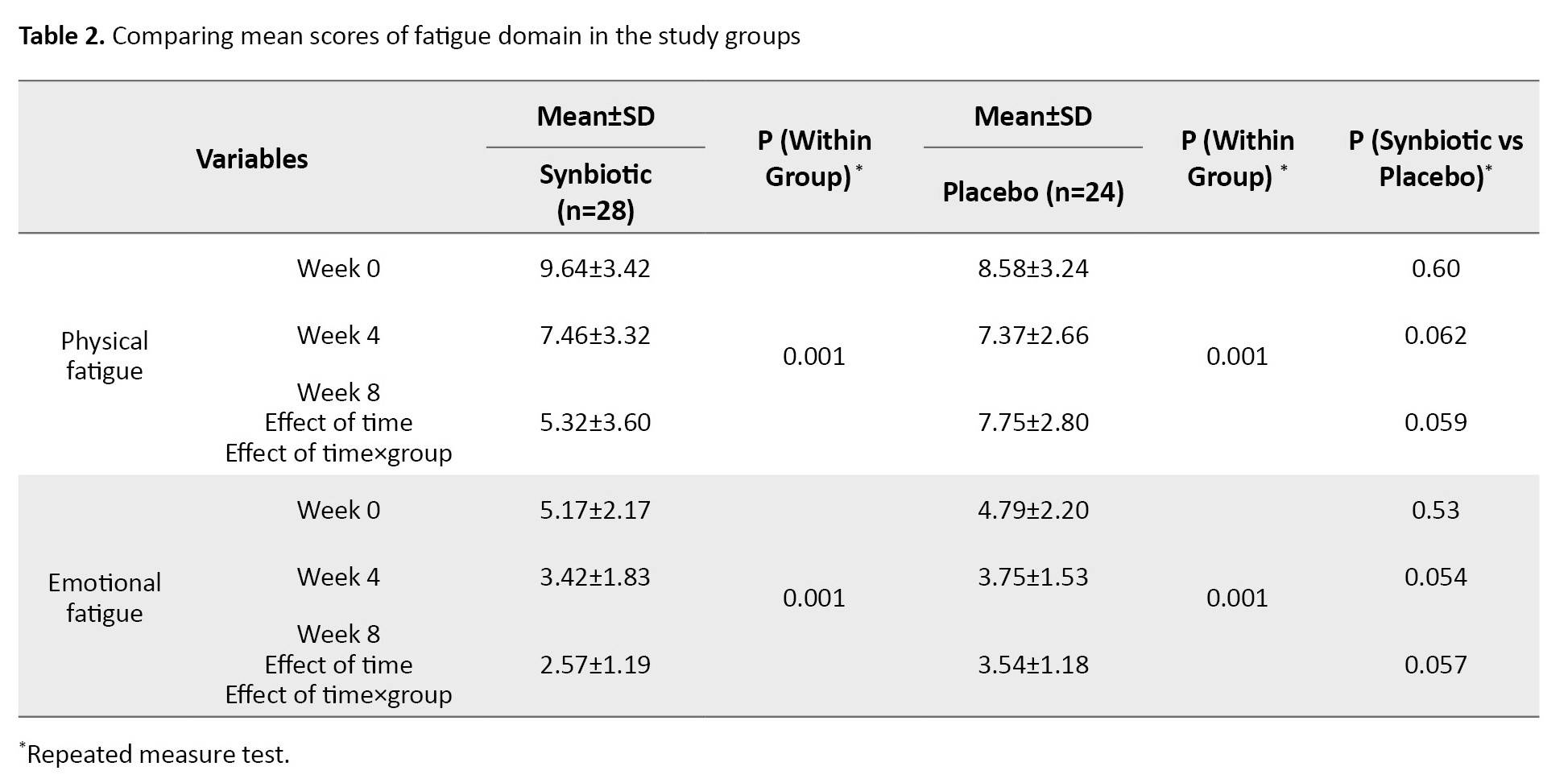
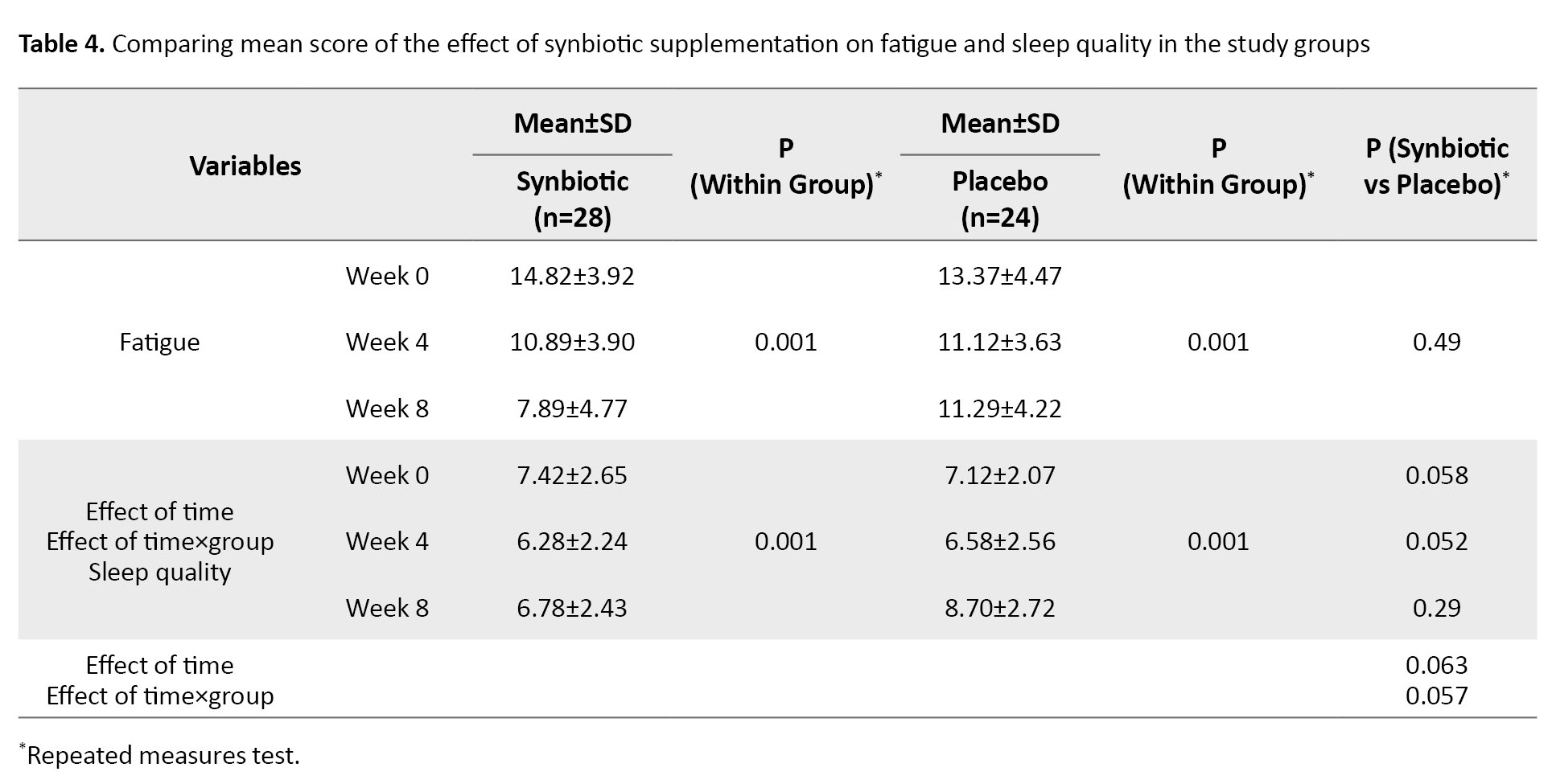
Regarding the CFS questionnaire, fatigue was investigated in two domains: Emotional and physical. The use of the synbiotic supplement in the intervention group led to a decrease in the level of fatigue in both domains of physical and emotional fatigue, and changes in the mean fatigue score were significant in both the intervention and placebo groups and in both physical and emotional fatigue domains (P=0.001). Still, this change was not significant between the groups. These results are shown in Table 2.
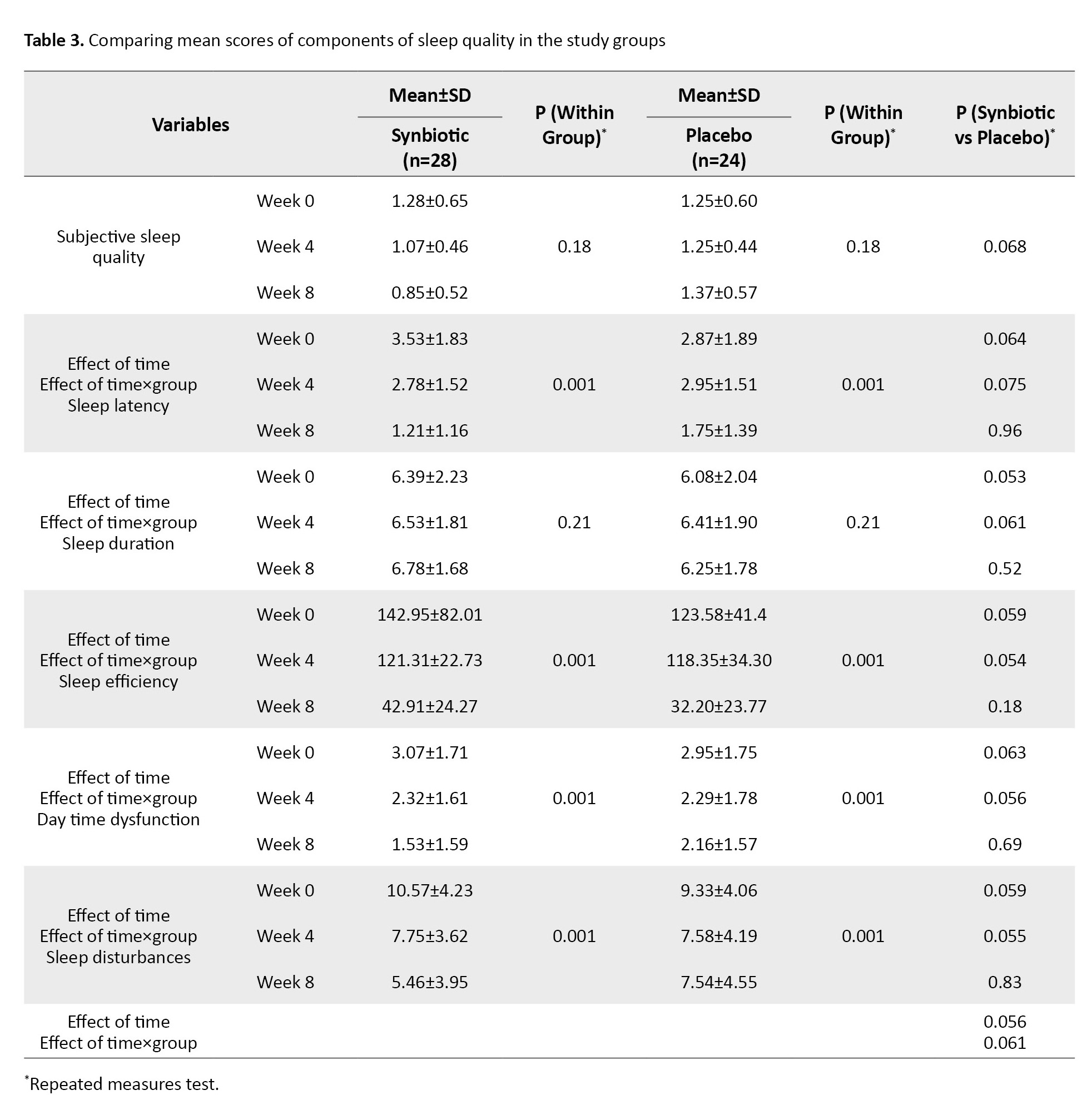
Regarding the PSQI questionnaire, sleep quality in patients was measured in 7 essential components (psychological sleep quality, latency of sleep, sleep duration, sleep efficiency, sleep disorders, use of sleeping pills, and inefficiency during the day). In the intervention group, taking synbiotic supplements for 8 weeks improved the quality of sleep (P=0.001), reduced daily functional disorders (P=0.001) and sleep disorders (P=0.001), and accelerated the start of the sleep cycle (P=0.001). However, this positive effect was not significant compared to the control group (Table 3).
None of the participants used sleeping pills during the study.
It was determined according to the results that the regular use of a synbiotic supplement for 8 weeks did not show a change in the amount of fatigue and sleep quality reported in patients receiving the synbiotic supplement considering the effect of time and group compared to the placebo group (Table 4).
Discussion
The daily intake of synbiotic supplements for 60 days was well tolerated in chronic renal failure patients under hemodialysis, and no complications developed. This research showed that the consumption of synbiotic supplements can significantly reduce fatigue in both physical and emotional domains and improve different domains of sleep quality, including acceleration of falling asleep, sleep quality, daily functional disorders, and sleep disorders in the intervention group. However, no significant difference was observed in the control group. Therefore, it cannot be said that the intervention of synbiotics alone causes the observed effect.
Lee et al.’s research showed that taking synbiotic supplements for 8 weeks improves the fatigue level of patients with irritable bowel syndrome. The difference in the findings may be related to the type of questionnaire used, the difference in the studied population, and the type of bacterial strains used [27]. Our study was similar to Talebi et al.’s study, which was conducted to investigate the effect of synbiotic supplements on the fatigue of patients with hypothyroidism. They showed that although the supplement improved general fatigue, these changes were not significant compared to the control group [28]. However, another study examining the general domains of QoL showed that using synbiotics does not affect sleep quality and fatigue in patients with chronic kidney disease [9]. In another study conducted by Kurdi et al. to evaluate the role of probiotics in regulating sleep and reducing stress in patients undergoing surgery, a non-invasive biomarker called salivary alpha-amylase was used. Compared between the groups, there was a significant decrease in perceived stress scores and salivary alpha-amylase. In addition, psychomotor alertness performance scores increased, indicating improved sleep in the pre-operative period in patients undergoing surgery [29]. The difference in the findings may be related to using more accurate tools to monitor sleep quality and a wider statistical population. In another study, sleep quality improvement was observed in the group receiving probiotics after 6 weeks, although these findings could not be assessed compared to the control group [30]. However, other studies have also shown that probiotic consumption can improve sleep quality in patients with chronic fatigue syndrome [31], chronic pain [32], and medical students under stress [33].
Despite the studies suggesting synbiotics to reduce fatigue and improve the quality of sleep in hemodialysis patients (because of the anti-inflammatory properties proven in synbiotic supplements) [18, 19], the present study only showed the anti-inflammatory effect of the supplement indirectly by examining sleep quality and fatigue in the intervention group. It can be said that it is better to conduct research in broader statistical communities and more accurate tools along with questionnaires, considering factors such as the complexity of the fatigue process and sleep quality, various physiological and environmental factors, and the course of the disease affecting patients treated with hemodialysis.
Synbiotic supplementation for 8 weeks has beneficial effects on fatigue and some aspects of sleep quality in patients with chronic kidney disease undergoing hemodialysis. However, these changes between the two study groups were not statistically significant. Synbiotic supplements can be considered a therapeutic approach to improve the symptoms of patients undergoing hemodialysis treatment. However, further studies with larger sample sizes and longer duration are suggested to confirm these findings.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Sabzevar University of Medical Sciences was responsible for following up on the participants’ reports regarding related issues and possible complications. Adverse events during the study were reported to the Medical Ethics Committee. At the beginning of the study, informed consent was obtained from the samples.
Written and oral instructions on using the capsules were provided on the first visit, and the study was conducted according to the guidelines of the Helsinki Declaration. All procedures involving human patients were approved by the Ethics Committee of Sabzevar University of Medical Sciences (Code: IR.MEDSAB.REC.1399.163). The study was registered by the Iranian Registry of Clinical Trials (IRCT) (No.: IRCT20210117050055N1)).
Funding
This study is taken from the master's thesis of Mina Jangpour, approved by Department of Nursing, Sabzevar University of Medical Sciences and was financially supported by Sabzevar University of Medical Sciences.
Authors' contributions
Study design and conceptualization: Mina Jangpour, Ali Asghar Jesmi, Akram Kooshki and Ali Taj; Methodology: Mina Jangpour and Ali Asghar Jesmi; Sampling: Mina Jangpour; Data analysis: Neda Mahdavifar; Writing the original daft: Akram Koshki and Ali Asghar Jesmi; Review and editing: Ali Tajabadi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Hemodialysis Unit of Musa bin Jafar, Qochan Hospital staff for their valuable assistance. The authors also gratefully acknowledge the samples that participated in this study.
References
Chronic kidney disease is one of the public health issues in the world. End-stage renal disease (ESRD) occurs when kidney function declines less than 10% to 15% of its normal level [1]. The prevalence of chronic kidney disease is estimated to be 8% to 16% worldwide [2]. In most developed countries, hemodialysis is the standard strategy for treatment at the beginning of ESRD and in patients over 65 years [3].
Fatigue is one of the most common complications experienced by hemodialysis patients. At least half of the patients complain of persistent fatigue, and about 86% report fatigue after dialysis [3, 4]. Also, about 50% to 83% of these patients suffer from poor sleep quality [5]. The onset of chronic kidney disease and hemodialysis, followed by complications such as fatigue and decreased sleep quality, significantly affect the patients’ quality of life (QoL) [6]. In a study conducted by Bossola et al. on fatigue in patients undergoing hemodialysis, fatigue unrelated to depression alone can increase the risk of mortality in these patients [7]. Factors such as anemia, inflammation, and hemodialysis may trigger the onset of the fatigue cycle and then the patients’ cognitive, behavioral, and emotional responses to early symptoms such as negative beliefs, depression, anxiety, and maladaptive behaviors. Gradually, this cycle leads to physiological complications such as systemic inflammation, central nervous system and endocrine disorders, and poor sleep quality. Finally, the fatigue cycle can lead to poor clinical outcomes in the patient [3].
Recently, the role of inflammatory factors as a primary agent in the fatigue cycle has become more prominent [8]. Therefore, it has been suggested that reducing systemic inflammation can effectively reduce fatigue, improve sleep quality, and ultimately improve the QoL [9, 10].
Scheper et al. introduced the intestine as a neglected organ in chronic kidney disease and its associated uremia [11]. Recent studies have shown that imbalances and quantitative and qualitative changes in the composition and activity of intestinal microbiota in ESRD patients accelerate the disease’s progression, uremia, and its complications, such as fatigue and poor sleep quality [12, 13]. High levels of potassium and phosphate in patients undergoing hemodialysis cause them to avoid the source of these elements. Consequently, patients are deprived of a rich source of indigestible complex carbohydrates, which are the main nutrients for intestinal bacteria [14]. The brain-intestine-kidney axis plays an essential role in the body’s normal homeostasis [15].
Probiotics are defined as beneficial living microorganisms if taken at an optimum level. Prebiotics are also described as dietary supplements (such as inulin) that support the growth of probiotics. Synbiotics are a combination of probiotics and prebiotics that may have a more significant effect on gastrointestinal health and systemic inflammation in hemodialysis patients due to their synergistic effect [9-14].
Therefore, it is hypothesized that changes in intestinal microbiota following synbiotic supplements can improve metabolic health by reducing inflammatory factors. Kooshki et al. reported that taking 8 weeks of synbiotic dietary supplement in hemodialysis patients can reduce inflammatory factors (C-reactive protein), malondialdehyde, total cholesterol, and low-density lipoprotein [16]. Also, considering the important role of anemia in chronic fatigue caused by chronic diseases, a systematic review and meta-analysis showed increasing iron absorption with probiotics consumption [17].
Considering that several studies have investigated the effect of synbiotic supplementation on fatigue, inflammatory and anemic factors, and improving the immune system of patients [18, 19], we evaluated the effects of synbiotic supplement therapy on improving sleep quality and fatigue in ESRD patients undergoing hemodialysis.
Materials and Methods
The study was a double-blind, randomized, placebo-controlled clinical trial. The required sample size in this project was based on the Srinivasa et al. study [9]. Considering the Mean±SD of fatigue scores before and after using synbiotic supplementation were reported as 42.76±22.22 and 47.50±21.87, respectively, α=0.05, and β=20% for each group, 26 people were obtained for each study group. This number increased to 30 people for each group, considering the possibility of 20% drop-out.
The 60 clinically stable hemodialysis (HD) patients aged 18 to 65 years receiving HD thrice weekly at the Dialysis Department of the Educational Center in Quchan, Iran, for at least 3 months before the onset of the study were enrolled. Dialysis duration was 3 to 4.5 hours per session, three times per week, with a blood flow of 250 mL/min and a dialysate flow of 500 mL/min.
The non-entry criteria based on the patient’s medical file included patients who had undergone kidney transplants or were likely to receive a transplant, those medically diagnosed with severe infections, diabetes, malignancy, chronic liver disease (hepatitis B, hepatitis C), gastrointestinal diseases (inflammatory bowel disease, ulcerative colitis, Crohn disease, celiac disease, lactose intolerance or allergy, irritable bowel syndrome), diabetic foot infection, pregnant or lactating mothers, and those with hemoglobin less than 8 mg/dL. According to the patient’s report, the Pittsburgh sleep quality index (PSQI) score is less than 6 (good sleep quality), the Chalder fatigue scale (CFS) score is less than 18, receive antioxidant vitamin supplements, prebiotics, probiotics, synbiotics, or antibiotics within one month of study (Figure 1). Demographic, CFS and PSQI questionnaires reported and measured patients’ characteristics, as well as fatigue and sleep quality. The CFS was first published by Chalder et al. This questionnaire contains 11 questions and two general domains for examining physical and emotional fatigue during the last month. Each question is assigned a score between 0 and 3; the total result is between 0 and 33. The global score also spans physical fatigue (measured by items 1–7) and psychological fatigue (measured by items 8–11). If the patient scores above 18, he or she will be considered tired; the patient suffers from more fatigue with a higher score [20]. In Iran, for the first time, Nasri et al. evaluated the validity of this questionnaire [21]. The fatigue tool’s validity was assessed using the content validity method, and its reliability was assessed using the internal validity method. The Cronbach α coefficient was calculated to be 0.79.
The PSQI is a self-report questionnaire designed by Buysse et al. and assesses sleep quality over the past month. The questionnaire includes 19 questions about 7 important components of sleep (mental quality of sleep, duration of sleep, duration of sleep, sleep efficiency, sleep disorders, use of sleeping pills, and inefficiency latency of sleep). Each component is assigned a score between 0 and 3, so the overall PSQI score is between 0 and 21. A score between 0 and 5 means good sleep quality, while a score between 6 and 21 means poor sleep quality [22]. This study used the psychometric Persian version of this questionnaire [23].
This research was a double-blind, randomized placebo study. The sampling method was convenient, and permuted block randomization was used to randomize the data. In this regard, 15 blocks with the size of four were formed (A and B for the intervention groups, C and D for the placebo group). Neither the patients, nephrologists, nor the researcher performing patients’ evaluation were aware of the group assignment. The baseline clinical evaluation included an anthropometric examination. Also, the PSQI and CFS questionnaires were administered to all patients. During the intervention, patients were asked to maintain a steady diet, engage in physical activity, take probiotic drugs, and not take supplements other than those provided by the trial.
The intervention group took two synbiotic capsules daily (one after lunch and the other after dinner), and the control group took two placebo capsules (containing 500 mg corn starch) daily at the same time for 8 weeks. These drugs are safe and have been used in similar studies [24-26]. Synbiotic and placebo capsules are similar in appearance and are marked only with the label “A” or “B” on the box. Boxes are tagged by a third party not directly involved in this study. Each Lactocore synbiotic capsule (500 mg) (produced by Zist Takhmir Company of Iran) contains high amounts of 12 beneficial and safe bacterial strains and fructooligosaccharide as a prebiotic. The strains used in this product include Lactobacillus rhamnosus, Lactobacillus Helveticus, Lactobacillus casei, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium breve, Lactobacillus bulgaricus, Bifidobacterium Langum, Lactobacillus plantarum, Bifidobacterium bifidum, Lactobacillus garii, and Streptococcus thermophilus. The placebo powder was comparable in color, texture, and taste to the synbiotics and prebiotics but contained only corn starch. Each box contained 30 capsules. The participants were given four boxes for the entire 2-month intervention (two boxes at the beginning of each month). In addition, the patients continued to take their daily medications during the study.
In subsequent visits, the patients were followed up by phone twice weekly to comply with the intervention. They were asked to take one capsule after lunch and one after dinner, which improved their adherence to the intervention. In addition, the samples were asked to record their daily intake of supplements or placebo in the notebook provided and to return any remaining capsules in the boxes at the next visit. At the end of 8 weeks, if the number of remaining capsules for each participant was more than 10% of the prescribed capsules, that participant was removed from the intervention.
Statistical analysis was performed using SPSS software, version 21 (Chicago, IL). Quantitative variables were described as Mean±SD, and qualitative variables as number (percentage). The repeated measures test was used because the outcome variable was measured three times in each group. Demographic variables were analyzed using the chi-square test, Fisher exact test, or independent sample t-test, as appropriate. Also, sphericity assumed Greenhouse-Geisser was used to check the homogeneity of the data. For all analyses, P<0.05 were considered statistically significant.
Results
Of 100 patients referred for hemodialysis treatment, 60 were included in the study and assigned to the synbiotic group (31 patients) and the placebo group (29 patients).
The data of 3 participants from the synbiotic group and 5 participants from the placebo group were excluded due to death after catching COVID-19 during the follow-up period. The remaining samples (n=52, 26 males and 26 females) were randomly allocated to the placebo (n=24, 12 males and 12 females) or the synbiotic supplement (n=28, 14 males and 14 females) groups.
Their mean age in the synbiotic group was 47.19±12.63 years, and in the placebo group, 48.79±11.01 years. All enrolled patients received erythropoietin and intravenous iron as a regular treatment following our regional HD guidelines (Table 1).

There were no significant side effects associated with the study treatments. However, some adverse events assessed as unrelated to study participation occurred among two patients in the placebo group: Oral mucositis and respiratory problems.
Regarding the CFS questionnaire, fatigue was investigated in two domains: Emotional and physical. The use of the synbiotic supplement in the intervention group led to a decrease in the level of fatigue in both domains of physical and emotional fatigue, and changes in the mean fatigue score were significant in both the intervention and placebo groups and in both physical and emotional fatigue domains (P=0.001). Still, this change was not significant between the groups. These results are shown in Table 2.

Regarding the PSQI questionnaire, sleep quality in patients was measured in 7 essential components (psychological sleep quality, latency of sleep, sleep duration, sleep efficiency, sleep disorders, use of sleeping pills, and inefficiency during the day). In the intervention group, taking synbiotic supplements for 8 weeks improved the quality of sleep (P=0.001), reduced daily functional disorders (P=0.001) and sleep disorders (P=0.001), and accelerated the start of the sleep cycle (P=0.001). However, this positive effect was not significant compared to the control group (Table 3).

None of the participants used sleeping pills during the study.
It was determined according to the results that the regular use of a synbiotic supplement for 8 weeks did not show a change in the amount of fatigue and sleep quality reported in patients receiving the synbiotic supplement considering the effect of time and group compared to the placebo group (Table 4).

Discussion
The daily intake of synbiotic supplements for 60 days was well tolerated in chronic renal failure patients under hemodialysis, and no complications developed. This research showed that the consumption of synbiotic supplements can significantly reduce fatigue in both physical and emotional domains and improve different domains of sleep quality, including acceleration of falling asleep, sleep quality, daily functional disorders, and sleep disorders in the intervention group. However, no significant difference was observed in the control group. Therefore, it cannot be said that the intervention of synbiotics alone causes the observed effect.
Lee et al.’s research showed that taking synbiotic supplements for 8 weeks improves the fatigue level of patients with irritable bowel syndrome. The difference in the findings may be related to the type of questionnaire used, the difference in the studied population, and the type of bacterial strains used [27]. Our study was similar to Talebi et al.’s study, which was conducted to investigate the effect of synbiotic supplements on the fatigue of patients with hypothyroidism. They showed that although the supplement improved general fatigue, these changes were not significant compared to the control group [28]. However, another study examining the general domains of QoL showed that using synbiotics does not affect sleep quality and fatigue in patients with chronic kidney disease [9]. In another study conducted by Kurdi et al. to evaluate the role of probiotics in regulating sleep and reducing stress in patients undergoing surgery, a non-invasive biomarker called salivary alpha-amylase was used. Compared between the groups, there was a significant decrease in perceived stress scores and salivary alpha-amylase. In addition, psychomotor alertness performance scores increased, indicating improved sleep in the pre-operative period in patients undergoing surgery [29]. The difference in the findings may be related to using more accurate tools to monitor sleep quality and a wider statistical population. In another study, sleep quality improvement was observed in the group receiving probiotics after 6 weeks, although these findings could not be assessed compared to the control group [30]. However, other studies have also shown that probiotic consumption can improve sleep quality in patients with chronic fatigue syndrome [31], chronic pain [32], and medical students under stress [33].
Despite the studies suggesting synbiotics to reduce fatigue and improve the quality of sleep in hemodialysis patients (because of the anti-inflammatory properties proven in synbiotic supplements) [18, 19], the present study only showed the anti-inflammatory effect of the supplement indirectly by examining sleep quality and fatigue in the intervention group. It can be said that it is better to conduct research in broader statistical communities and more accurate tools along with questionnaires, considering factors such as the complexity of the fatigue process and sleep quality, various physiological and environmental factors, and the course of the disease affecting patients treated with hemodialysis.
Synbiotic supplementation for 8 weeks has beneficial effects on fatigue and some aspects of sleep quality in patients with chronic kidney disease undergoing hemodialysis. However, these changes between the two study groups were not statistically significant. Synbiotic supplements can be considered a therapeutic approach to improve the symptoms of patients undergoing hemodialysis treatment. However, further studies with larger sample sizes and longer duration are suggested to confirm these findings.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Sabzevar University of Medical Sciences was responsible for following up on the participants’ reports regarding related issues and possible complications. Adverse events during the study were reported to the Medical Ethics Committee. At the beginning of the study, informed consent was obtained from the samples.
Written and oral instructions on using the capsules were provided on the first visit, and the study was conducted according to the guidelines of the Helsinki Declaration. All procedures involving human patients were approved by the Ethics Committee of Sabzevar University of Medical Sciences (Code: IR.MEDSAB.REC.1399.163). The study was registered by the Iranian Registry of Clinical Trials (IRCT) (No.: IRCT20210117050055N1)).
Funding
This study is taken from the master's thesis of Mina Jangpour, approved by Department of Nursing, Sabzevar University of Medical Sciences and was financially supported by Sabzevar University of Medical Sciences.
Authors' contributions
Study design and conceptualization: Mina Jangpour, Ali Asghar Jesmi, Akram Kooshki and Ali Taj; Methodology: Mina Jangpour and Ali Asghar Jesmi; Sampling: Mina Jangpour; Data analysis: Neda Mahdavifar; Writing the original daft: Akram Koshki and Ali Asghar Jesmi; Review and editing: Ali Tajabadi; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Hemodialysis Unit of Musa bin Jafar, Qochan Hospital staff for their valuable assistance. The authors also gratefully acknowledge the samples that participated in this study.
References
- Roshanzadeh M, Shirani M, Tajabadi A, Shirvani M, Mohammadi S. [The effect of music and movie-watching on hemodynamic parameters of patients undergoing extracorporeal lithotripsy (Persian)]. J Hayat. 2021; 27(4):401-15. [Link]
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. chronic kidney disease: Global dimension and perspectives. Lancet. 2013; 382(9888):260-72. [DOI:10.1016/S0140-6736(13)60687-X] [PMID]
- Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. A prospective study of fatigue trajectories among in-centre haemodialysis patients. Br J Health Psychol. 2020; 25(1):61-88. [DOI:10.1111/bjhp.12395] [PMID] [PMCID]
- Shureshi P, Ahmadi Chenari H, Ahmadi M, Jesmi AA. [Effect of education by lecture and pamphlet methods on soldiers knowledge about meningitis disease (Persian)]. J Mil Med. 2015; 17(3):181-6. [Link]
- Jamalinik M, Haddadi M, Abedi A, Tajabadi A, Ganjloo J, Hasheminik M. [COVID-19; Symptoms,transmission methods, care and treatment techniques based on the latest evidence available: A narrative review study (Persian)]. Iran Occup Health. 2020; Special Issue: Covid-19:1-14. [Link]
- Davarinia Motlagh Quchan A, Tajabadi A, Borzoee F, Heshmatifar N, Mohamadzadeh Tabrizi Z, Rastaghi S. [Comparison of mental health of nurses working in Covid-19 reference hospitals with other hospitals (Persian)]. J Mil Med. 2020; 22(11):1145-52. [Link]
- Bossola M, Di Stasio E, Antocicco M, Panico L, Pepe G, Tazza L. Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron. 2015; 130(2):113-8. [DOI:10.1159/000430827] [PMID]
- Bossola M, Luciani G, Tazza L. Fatigue and its correlates in chronic hemodialysis patients. Blood Purif. 2009; 28(3):245-52. [DOI:10.1159/000231985] [PMID]
- Srinivasa S, Madhusudhan SK. A prospective study to evaluate the safety and efficacy of symbiotic supplementation (probiotic and prebiotic combination) in stage 5D chronic kidney disease patients. Int J Basic Clin Pharmacol. 2017; 6(4):765-73. [Link]
- Erten Y, Kokturk O, Yuksel A, Elbeg S, Ciftci TU, Pasaoglu H, et al. Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology. 2005; 10(4):330-5. [DOI:10.1111/j.1440-1797.2005.00418.x] [PMID]
- Schepers E, Glorieux G, Vanholder R. The gut: The forgotten organ in uremia? Blood Purif. 2010; 29(2):130-6. [DOI:10.1159/000245639] [PMID]
- Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018; 14(7):442-56. [DOI:10.1038/s41581-018-0018-2] [PMID] [PMCID]
- Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: A potential therapeutic target. Am J Kidney Dis. 2016; 67(3):483-98. [DOI:10.1053/j.ajkd.2015.09.027] [PMID] [PMCID]
- Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effects of synbiotics and probiotics supplementation on serum levels of endotoxin, heat shock protein 70 antibodies and inflammatory markers in hemodialysis patients: A randomized double-blinded controlled trial. Probiotics Antimicrob Proteins. 2020; 12(1):144-51. [DOI:10.1007/s12602-018-9509-5] [PMID]
- Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: A randomized, double-blinded, clinical trial. Nutr Neurosci. 2021; 24(6):490-9. [DOI:10.1080/1028415X.2019.1646975] [PMID]
- Kooshki A, Tofighiyan T, Miri M. A synbiotic supplement for inflammation and oxidative stress and lipid abnormalities in hemodialysis patients. Hemodial Int. 2019; 23(2):254-60. [DOI:10.1111/hdi.12748] [PMID]
- Vonderheid SC, Tussing-Humphreys L, Park C, Pauls H, OjiNjideka Hemphill N, LaBomascus B, et al. A systematic review and meta-analysis on the effects of probiotic species on iron absorption and iron status. Nutrients. 2019; 11(12):2938. [DOI:10.3390/nu11122938] [PMID] [PMCID]
- Irwin C, McCartney D, Desbrow B, Khalesi S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: A systematic review and meta-analysis. Eur J Clin Nutr. 2020; 74(11):1536-49. [DOI:10.1038/s41430-020-0656-x] [PMID]
- Mirzaeian S, Saraf-Bank S, Entezari MH, Hekmatdoost A, Feizi A, Atapour A. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: A double-blind, placebo-controlled, randomized clinical trial. Nutrition. 2020; 73:110713. [DOI:10.1016/j.nut.2019.110713] [PMID]
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993; 37(2):147-53. [DOI:10.1016/0022-3999(93)90081-P] [PMID]
- Nasri S. [Epidemiological study of chronic fatigue syndrome and its relation to psychiatric difficulties in nurses (Persian)]. Iran J Psychiatry Clin Psychol. 2004; 9(4):25-33. [Link]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2):193-213. [DOI:10.1016/0165-1781(89)90047-4] [PMID]
- Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012; 16(1):79-82. [DOI:10.1007/s11325-010-0478-5] [PMID]
- Rodrigues HCN, Martins TFP, Santana NCFES, Braga CC, Silva MAC, Cunha LCD, et al. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin Nutr ESPEN. 2021; 44:136-42. [DOI:10.1016/j.clnesp.2021.06.006] [PMID]
- Borges NA, Sahiun E, Ribeiro-Alves M, Regis B, Mafra D. Effects of Polydextrose Supplementation on Intestinal Function in Hemodialysis Patients: A Double-Blind, Randomized, Placebo-Controlled Trial. J Ren Nutr. 2023; 33(6):747-54. [DOI:10.1053/j.jrn.2023.06.008] [PMID]
- Hosseini R, Montazerifar F, Shahraki E, Karajibani M, Mokhtari AM, Dashipour AR, et al. The effects of zinc sulfate supplementation on serum copeptin, C-Reactive protein and metabolic markers in zinc-deficient diabetic patients on hemodialysis: A randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2022; 200(1):76-83. [DOI:10.1007/s12011-021-02649-7] [PMID]
- Lee SH, Cho DY, Lee SH, Han KS, Yang SW, Kim JH, et al. A randomized clinical trial of synbiotics in irritable bowel syndrome: Dose-dependent effects on gastrointestinal symptoms and fatigue. Korean J Fam Med. 2019; 40(1):2-8. [DOI:10.4082/kjfm.17.0064] [PMID] [PMCID]
- Talebi S, Karimifar M, Heidari Z, Mohammadi H, Askari G. The effects of synbiotic supplementation on fatigue management and mental health status in levothyroxine-treated patients with hypothyroidism: A randomized, double-blind, placebo-controlled clinical trial. J Isfahan Med Sch. 2016; 38(564):74-84. [DOI:10.22122/JIMS.V38I564.12428]
- Kurdi MS, Ramaswamy AH, Kumar LA, Choukimath SM, Jangi AA. Use of a non-invasive biomarker salivary alpha-amylase to assess the role of probiotics in sleep regulation and stress attenuation in surgical patients: A randomised double-blind clinical trial. Indian J Anaesth. 2021; 65(5):390-7. [DOI:10.4103/ija.IJA_1498_20] [PMID] [PMCID]
- Marotta A, Sarno E, Del Casale A, Pane M, Mogna L, Amoruso A, et al. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psychiatry. 2019; 10:164. [DOI:10.3389/fpsyt.2019.00164] [PMID] [PMCID]
- Jackson ML, Butt H, Ball M, Lewis DP, Bruck D. Sleep quality and the treatment of intestinal microbiota imbalance in chronic fatigue syndrome: A pilot study. Sleep Sci. 2015; 8(3):124-33. [DOI:10.1016/j.slsci.2015.10.001] [PMID] [PMCID]
- Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes. 2016; 7(2):153-6.[DOI:10.3920/BM2015.0100] [PMID]
- Sawada D, Kawai T, Nishida K, Kuwano Y, Fujiwara S, Rokutan K. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J Funct Foods. 2017; 31:188-97. [DOI:10.1016/j.jff.2017.01.042]
Article Type : Research |
Subject:
General
Received: 2022/12/14 | Accepted: 2023/12/18 | Published: 2024/07/1
Received: 2022/12/14 | Accepted: 2023/12/18 | Published: 2024/07/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |