Tue, Feb 3, 2026
Volume 35, Issue 2 (3-2025)
JHNM 2025, 35(2): 149-157 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pouy S, Taheri_Ezbarami Z, Rassouli M, Maroufizadeh S, Darbandi B, Javadi-Pashaki N. Translation and Psychometric Evaluation of the Persian Version of the Pediatric Quality of Life Inventory 4.0 (PedsQL 4.0) for Iranian Children With Cancer. JHNM 2025; 35 (2) :149-157
URL: http://hnmj.gums.ac.ir/article-1-2362-en.html
URL: http://hnmj.gums.ac.ir/article-1-2362-en.html
Somaye Pouy1 

 , Zahra Taheri_Ezbarami2
, Zahra Taheri_Ezbarami2 

 , Maryam Rassouli3
, Maryam Rassouli3 

 , Saman Maroufizadeh4
, Saman Maroufizadeh4 

 , Bahram Darbandi5
, Bahram Darbandi5 

 , Nazila Javadi-Pashaki *6
, Nazila Javadi-Pashaki *6 




 , Zahra Taheri_Ezbarami2
, Zahra Taheri_Ezbarami2 

 , Maryam Rassouli3
, Maryam Rassouli3 

 , Saman Maroufizadeh4
, Saman Maroufizadeh4 

 , Bahram Darbandi5
, Bahram Darbandi5 

 , Nazila Javadi-Pashaki *6
, Nazila Javadi-Pashaki *6 


1- Assistant Professor, Department of Nursing, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
2- Associate Professor, Department of Nursing, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
3- Professor, Cancer Research Center, School of Nursing & Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Assistant professor, Department of Biostatistics and Epidemiology, School of Health, Guilan University of Medical Sciences, Rasht, Iran.
5- Associate Professor, Pediatric Diseases Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
6- Professor, Social Determinants of Health Research Center (SDHRC), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. ,n.javadip@gmail.com
2- Associate Professor, Department of Nursing, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
3- Professor, Cancer Research Center, School of Nursing & Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Assistant professor, Department of Biostatistics and Epidemiology, School of Health, Guilan University of Medical Sciences, Rasht, Iran.
5- Associate Professor, Pediatric Diseases Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
6- Professor, Social Determinants of Health Research Center (SDHRC), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. ,
Keywords: Pediatric quality of life inventory (PedsQL), Children, Health-related quality of life (HRQOL), Cancer, Iran
Full-Text [PDF 622 kb]
(670 Downloads)
| Abstract (HTML) (853 Views)
Full-Text: (285 Views)
Introduction
Cancer is one of the life-threatening diseases that affects the lives of many children in the world [1-3]. Cancer affects about 50-200 children per million worldwide [4]. In Iran, 19,973 children have been diagnosed with cancer in the recent ten years [5]. Cancer is one of the leading causes of death for children worldwide [6-8]. To reduce the cancer death rate in children, there are many methods such as chemotherapy, bone marrow transplant, and radiotherapy [9-11]. Despite the usefulness of these methods, they have many side effects for children, which affect their health-related quality of life (HRQOL) [12]. HRQOL is an assessment of how a person’s well-being is affected over time by a disease, disability or disorder [13]. In children with cancer, due to the impact of the disease on various aspects of their lives, HRQOL assessment is very important [14]. Nowadays, more than 80% of children with cancer survive [15, 16]; therefore, special attention should be paid to their HRQOL [17].
To assess HRQOL in children with cancer, it is necessary to have an appropriate instrument that can assess all physical, psychological and social aspects. This tool should also include child and parent feedback and consider the child’s cognitive development [18-20]. There are few tools for assessment such as the Minneapolis-Manchester Quality of Life Instrument and the pediatric quality of life (PedsQL 4.0) [21, 22]. The PedsQL 4.0 is a suitable instrument for evaluating HRQOL in children, which considers all the mentioned criteria [23]. This tool consists of two parts; one part is the child self-report for ages 5-18 and the other part is the parent proxy-report for ages 2-18 years [23-25]. So far, this tool has been translated into many languages and psychometrically evaluated in different countries, but it has not yet been translated into Persian. Therefore, the present study aimed to determine the psychometric properties of the Persian version of the PedsQL 4.0 for Iranian children with cancer.
Methods
In this methodological study, the population includes the children with cancer and their parents referred to the Pediatric Oncology Department of a specialized hospital for children affiliated to Guilan University of Medical Sciences in Rasht, Iran, from October 2021 to October 2022. Inclusion criteria for children were age 2-18 years, a definite diagnosis of cancer by the physician for children who were in the active or follow-up treatment phase, and willingness of the child and informed consent of their parents to participate in the study. The children who were not physically or mentally stable (based on the physician’s diagnosis) were excluded. Inclusion criteria for parents were the ability to speak Persian, willingness to participate in the study, no mental disorders or a history of hospitalization in psychiatric wards. In general, at least 200 samples are recommended to perform factor analysis according to the literature. This sample size is able to provide a high test power of 0.80 for a model with 100 degrees of freedom [26]. Keeping this in mind, we included 200 children aged 2-18 years and 200 parents.
The PedsQL 4.0 has 27 items and eight subscales, including pain and hurt (2 items), nausea (5 items), procedural anxiety (3 items), treatment anxiety (3 items), worry (3 items), cognitive problems (5 items), perceived physical appearance (3 items) and communication (3 items). The child self-report is different based on age of the child (age 5-7, 8-12 and 13-18 years old). The parent proxy-report is also different based on the age of the child (age 2-4, 5-7, 8-12 and 13-18 years). The child self-report version uses a 3-point Likert scale (0 = not at all, 2 = sometimes, 4 a lot) for children aged 5-7 years, while a 5-point Likert scale (0=never, 1=almost never, 2=sometimes, 3=often, 4=almost always) is used for children aged 8-18 years and for the parent proxy-report version.
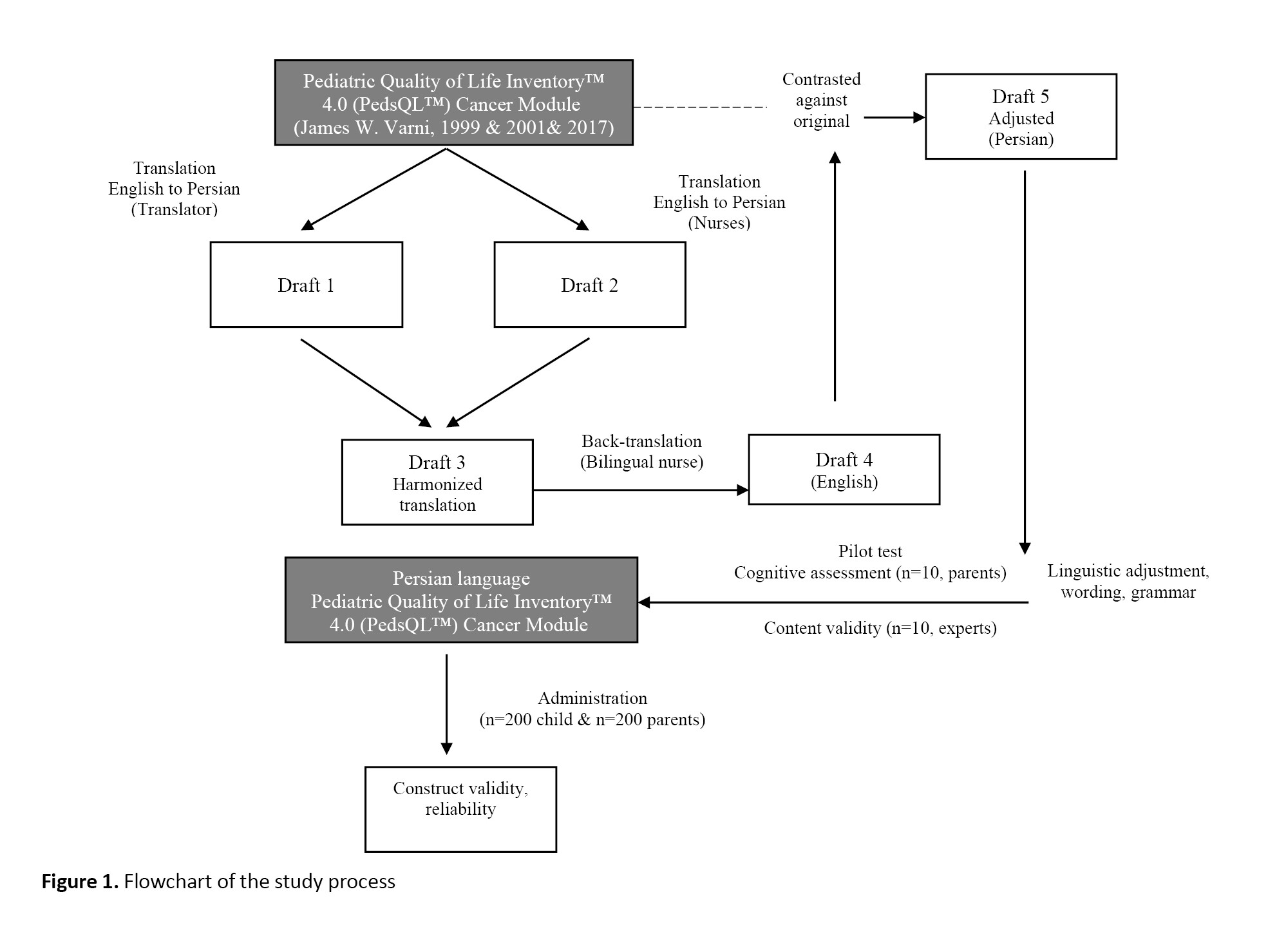
Translation and cross-cultural adaptation were first done based on Beaton et al.’s four-step approach. For this purpose, after correspondence with the developer of the main version (Dr. James W. Varni) [25] and obtaining permission from him, the forward and backward translation was carried out. First, an Iranian translator and a nurse familiar with English translated the questionnaire from English to Persian. Then, an initial Persian draft was obtained after checking the quality of translations and making modifications. A nurse familiar with English back-translated it into English. To compare the translated English draft with the original version of the questionnaire, it was emailed to Dr. Varni. Finally, the final Persian draft was approved after revising and editing. The flowchart of the study process is presented in Figure 1.
Parents were asked to complete both parent proxy-report (for ages 2-18) and child self-report (for ages 2-4) versions. The versions for 5-18 years old were completed by children. The information for parents (including age, relationship with the child, education and economic status) and children (including age, sex, type of cancer, and disease status) were also recorded. During the completion of the questionnaires, the researcher was available and answered their questions. The average time to complete the questionnaires was 20 minutes.
For content validity assessment, 10 experts in the fields of oncology, pediatric nursing, and psychometrics were asked to give their opinions about the grammar, wording, item allocation, and compatibility of the final draft with Iranian culture. The feasibility and comprehensibility of the items were assessed by 10 parents of children with cancer.
To determine the construct validity, confirmatory factor analysis (CFA) was used. Maximum likelihood estimation was used to estimate the factor matrix. Several fit indices are used in CFA, including χ2/df, χ2, df, goodness of fit index (GFI), adjusted goodness of fit (AGFI), comparative fit index (CFI), root mean square residuals (RMSR), root mean square error of approximation (RMSEA), and normed fit index (NFI). The acceptable values were considered as χ2/df <5, CFI >0.9 , GFI >0.9, AGFI >0.9, and RMSEA <0.08 [26, 27]. Considering the studies assessed the different versions of the PedsQL 4.0, a sample size of 200 children and 200 parents was determined to perform the CFA. To determine the internal consistency of the Persian version, the questionnaire was given to 200 participants, and Cronbach’s α coefficient was calculated. To determine the test re-test reliability, the questionnaire was completed by 50 children aged 2-18 and their parents (n=50), and the Intraclass correlation coefficient (ICC) was calculated. Statistical analyses were done in SPSS software, version 21. Descriptive statistics (frequency, percentage, Mean±SD) were used to describe the data, to perform CFA, LISREL software, version 8.8 was used.
Results
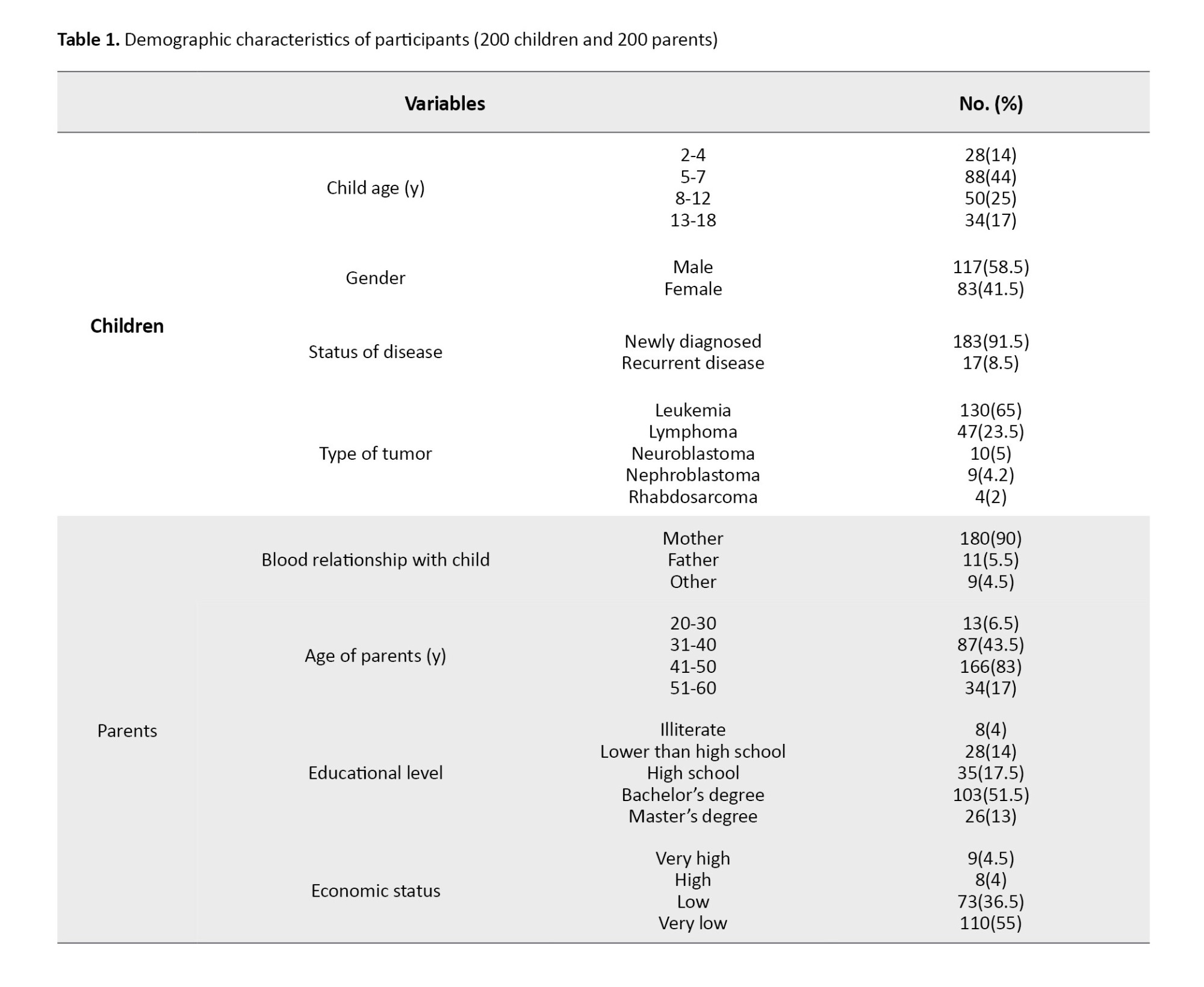
The age of children and their parents was 5.9±4.2 and 37.83±5.86 years, respectively. Other demographic and clinical characteristics are presented in Table 1.
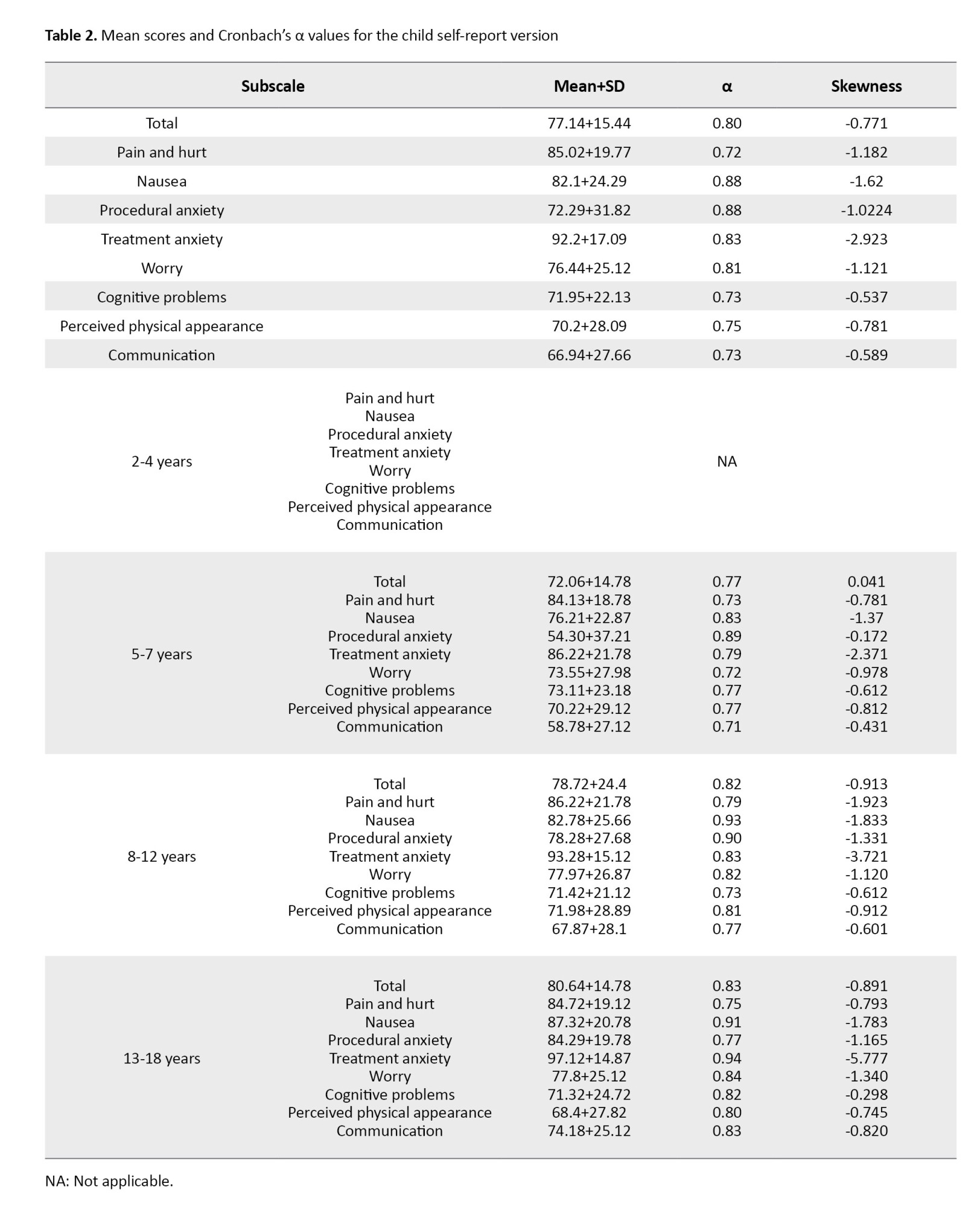
In the child self-report version, Cronbach’s α value for the eight subscales was in the range of 0.72-0.88 and for the overall scale, it was 0.80 (Table 2).
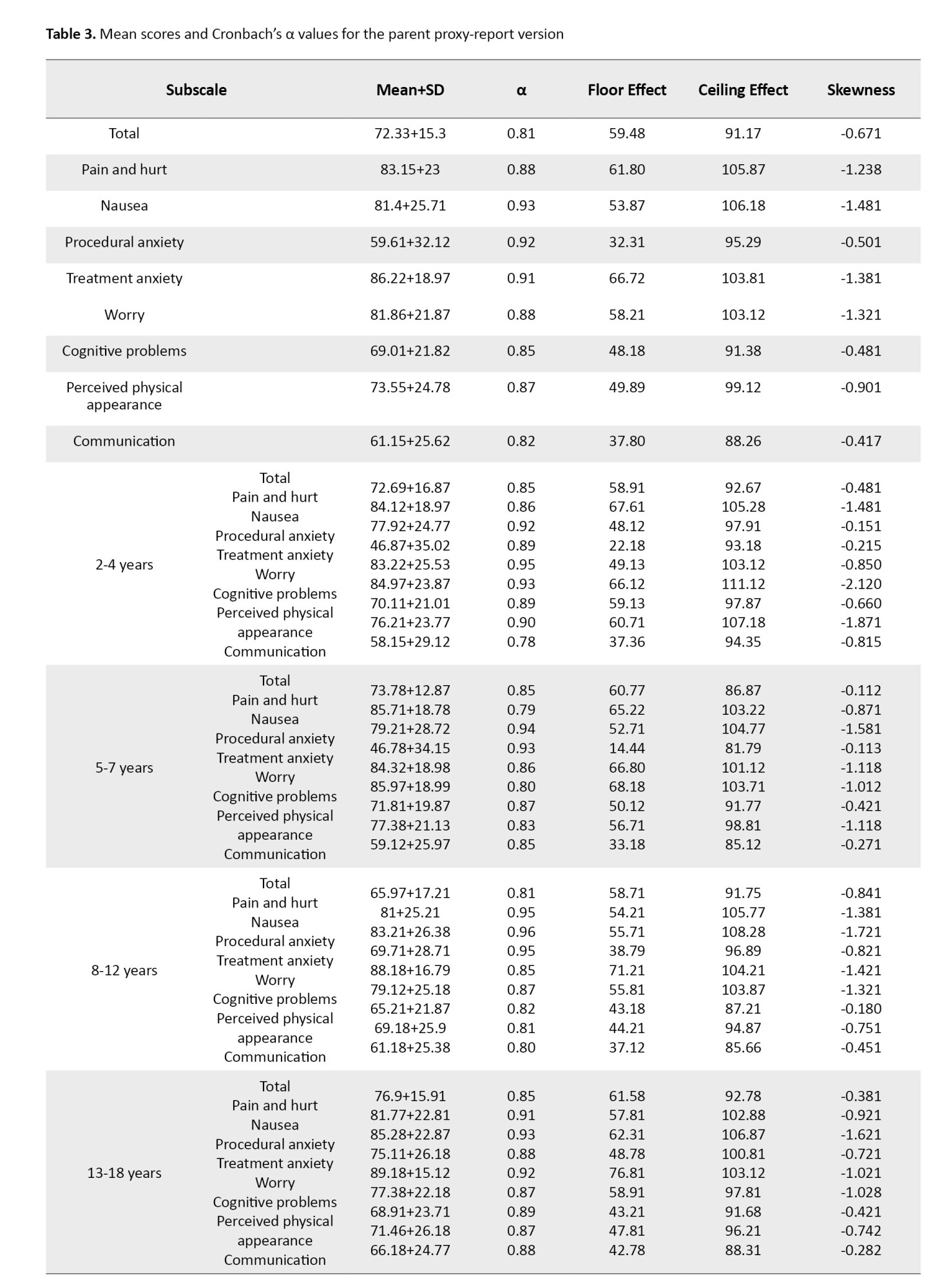
In the parent proxy-report version, Cronbach’s α value was in the range of 0.82-0.93 and for the overall scale, it was 0.81 (Table 3).
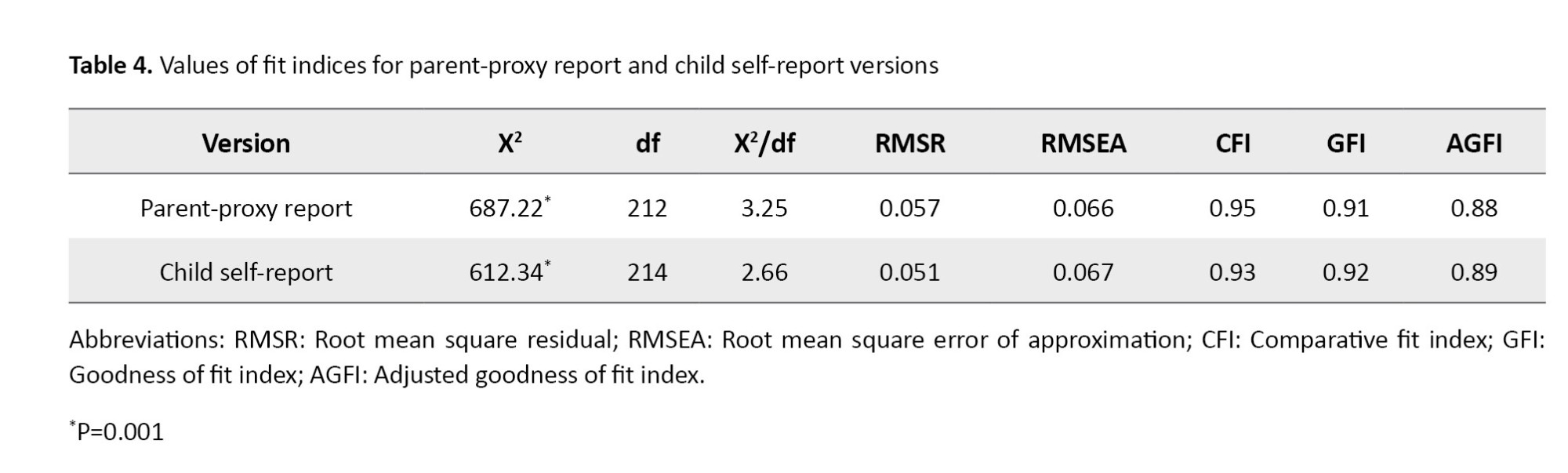
In determining the construct validity, all fit indices had acceptable value and confirmed the fitness of the final model (Table 4). Since the values were above 0.7 in both versions, it can be said that the Persian version had acceptable internal consistency.
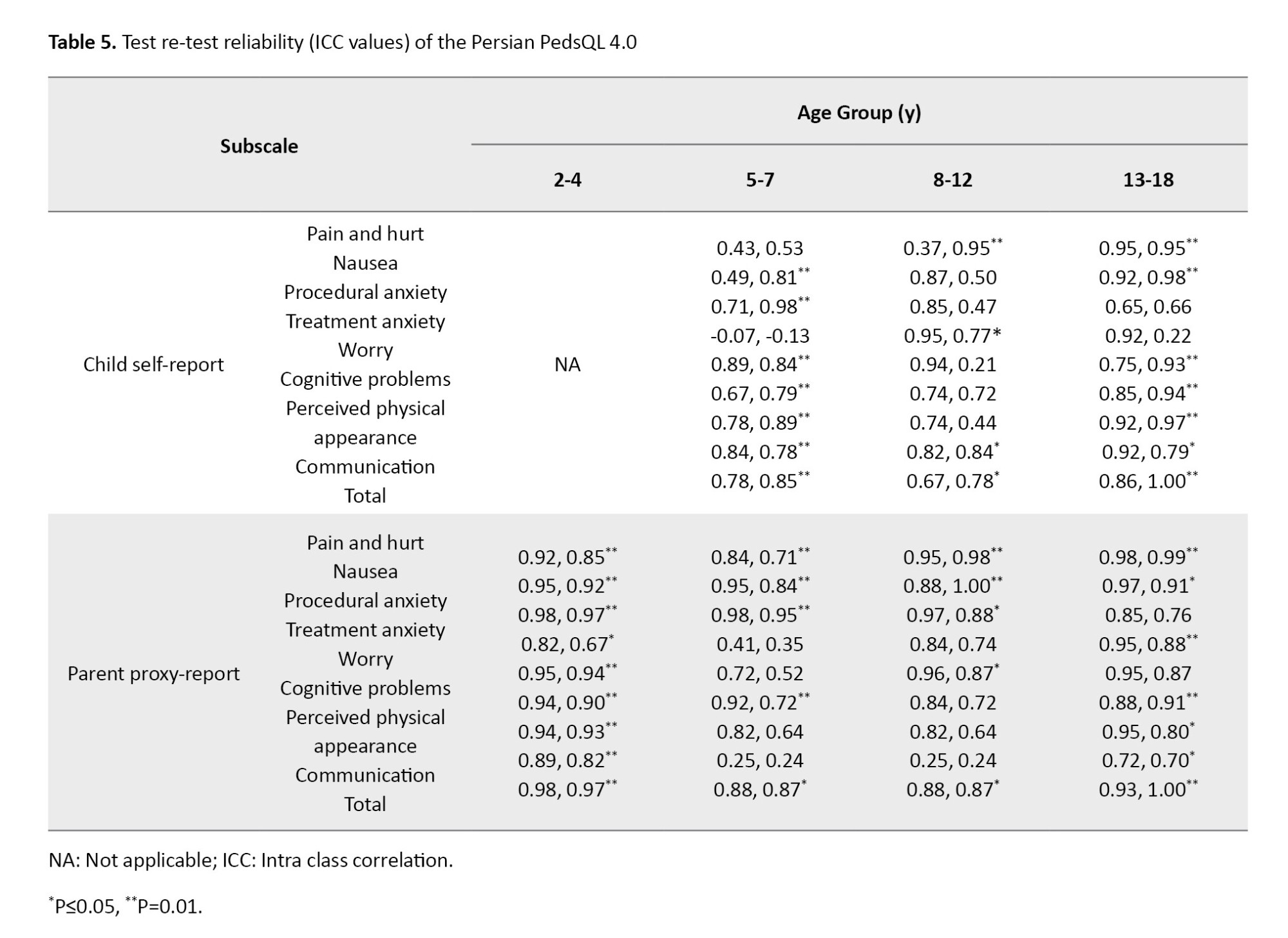
For the test re-test reliability, the ICC values for each age group of children are presented in Table 5. Except for the treatment anxiety subscale in the 5-7 years age group and the worry subscale in the 13-18 years age group, the ICC values were good to excellent. The ICC values for the parent proxy-report version also were good to excellent .
Discussion
The present study investigated the psychometric properties of the Persian version of the PedsQL 4.0 for Iranian children with cancer. The results showed the appropriate reliability and validity of the Persian PedsQL 4.0. Construct validity in this research was done using the CFA. The main version of the PedsQL 4.0 cancer has eight factors [23]. Our results showed that the Persian version (for both child self-report and parent proxy-report) had a good fit to the data and the eight-factor model was confirmed.
According to the Cronbach’s α values, all subscales of the Persian PedsQL 4.0 in the child self-report and parent proxy-report versions for all age groups had good or acceptable internal consistency. In line with the present study, the results of a psychometric study on the Chinese version of the PedsQL™ 4.0 showed that the Cronbach’s α values for the whole scale and the subscales in the child self-report and parent proxy-report were at an acceptable level and had a good internal consistency [28]. For the Brazilian version, a study showed that the Cronbach’s α values for the child and parent versions were at a acceptable level, but in contrast to our study, most of the subscales were not at a good level [29]. For the original version, Cronbach’s α values for the subscales of “perceived physical appearance” and “communication” in the children self-report were not at acceptable level [23].
The results of test re-test reliability assessment showed that except for the “treatment anxiety” subscale for 5-7 year age group and the “worry” subscale for the 13-18 year age group, the ICC values for all subscales of child and parent versions and different age groups were good to excellent. For the Japanese version, the ICC values of the subscale “treatment anxiety” in children aged 5-7 and 13-18 years old and the subscale “worry” for children aged 8-12 years were in the low range [30]. For the Chinese version, the ICC values for all subscales of child and parent versions were in the good to excellent range [28]. In the Brazilian version, the ICC was not calculated separately for different age groups, but they were in the appropriate range for the whole scale [29]. The low ICC of the “treatment anxiety” subscale for 5-7 year age group in our study can be due to the difficulty of explaining treatment anxiety by the items in this subscale for young children. The low ICC of the “worry” subscale for children aged 13-18 years may be due to the difference in treatments during two weeks, according to the items in this subscale. In this study, data collection was done in one hospital in northern Iran, which can affect the generalizability of the results to other centers.
In conclusion, the Persian version of the PedsQL™ 4.0 has acceptable psychometric properties and can be used as a valid and reliable tool to assess the HRQOL of Iranian children with cancer. It can help researchers and physicians in Iran to better understand the experiences of children with cancer and create more effective interventions to improve their quality of life. It is recommended that future studies focus on the sensitivity and responsiveness of this tool and provide the results to compare data for healthy children.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1400.267). The participants were free to leave the study at any time and their information was kept confidential. Written informed consent was obtained from parents and verbal consent from children to participate in the study.
Funding
This study was extracted from a PhD dissertation of Somaye Pouy, approved by the Department of Nursing, Faculty of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. this study was financially supported by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
Conceptualization, design, and resources: Somaye Pouy, Zahra Taheri Ezbarami, Maryam Rassouli and Nazila Javadi-Pashaki; Data collection: Somaye Pouy, and Saman Maroufizadeh; Data analysis: Nazila Javadi-Pashaki, Somaye Pouy and Saman Maroufizadeh; Investigation, and writing the original draft: Somaye Pouy, Zahra Taheri Ezbarami, Maryam Rassouli and Nazila Javadi-Pashaki; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research and Technology of Guilan University of Medical Sciences and the officials and medical staff of the selected specialized hospitals for children for supporting this study, and all children and their parents who participated in this study for their cooperation.
References
Cancer is one of the life-threatening diseases that affects the lives of many children in the world [1-3]. Cancer affects about 50-200 children per million worldwide [4]. In Iran, 19,973 children have been diagnosed with cancer in the recent ten years [5]. Cancer is one of the leading causes of death for children worldwide [6-8]. To reduce the cancer death rate in children, there are many methods such as chemotherapy, bone marrow transplant, and radiotherapy [9-11]. Despite the usefulness of these methods, they have many side effects for children, which affect their health-related quality of life (HRQOL) [12]. HRQOL is an assessment of how a person’s well-being is affected over time by a disease, disability or disorder [13]. In children with cancer, due to the impact of the disease on various aspects of their lives, HRQOL assessment is very important [14]. Nowadays, more than 80% of children with cancer survive [15, 16]; therefore, special attention should be paid to their HRQOL [17].
To assess HRQOL in children with cancer, it is necessary to have an appropriate instrument that can assess all physical, psychological and social aspects. This tool should also include child and parent feedback and consider the child’s cognitive development [18-20]. There are few tools for assessment such as the Minneapolis-Manchester Quality of Life Instrument and the pediatric quality of life (PedsQL 4.0) [21, 22]. The PedsQL 4.0 is a suitable instrument for evaluating HRQOL in children, which considers all the mentioned criteria [23]. This tool consists of two parts; one part is the child self-report for ages 5-18 and the other part is the parent proxy-report for ages 2-18 years [23-25]. So far, this tool has been translated into many languages and psychometrically evaluated in different countries, but it has not yet been translated into Persian. Therefore, the present study aimed to determine the psychometric properties of the Persian version of the PedsQL 4.0 for Iranian children with cancer.
Methods
In this methodological study, the population includes the children with cancer and their parents referred to the Pediatric Oncology Department of a specialized hospital for children affiliated to Guilan University of Medical Sciences in Rasht, Iran, from October 2021 to October 2022. Inclusion criteria for children were age 2-18 years, a definite diagnosis of cancer by the physician for children who were in the active or follow-up treatment phase, and willingness of the child and informed consent of their parents to participate in the study. The children who were not physically or mentally stable (based on the physician’s diagnosis) were excluded. Inclusion criteria for parents were the ability to speak Persian, willingness to participate in the study, no mental disorders or a history of hospitalization in psychiatric wards. In general, at least 200 samples are recommended to perform factor analysis according to the literature. This sample size is able to provide a high test power of 0.80 for a model with 100 degrees of freedom [26]. Keeping this in mind, we included 200 children aged 2-18 years and 200 parents.
The PedsQL 4.0 has 27 items and eight subscales, including pain and hurt (2 items), nausea (5 items), procedural anxiety (3 items), treatment anxiety (3 items), worry (3 items), cognitive problems (5 items), perceived physical appearance (3 items) and communication (3 items). The child self-report is different based on age of the child (age 5-7, 8-12 and 13-18 years old). The parent proxy-report is also different based on the age of the child (age 2-4, 5-7, 8-12 and 13-18 years). The child self-report version uses a 3-point Likert scale (0 = not at all, 2 = sometimes, 4 a lot) for children aged 5-7 years, while a 5-point Likert scale (0=never, 1=almost never, 2=sometimes, 3=often, 4=almost always) is used for children aged 8-18 years and for the parent proxy-report version.
Translation and cross-cultural adaptation were first done based on Beaton et al.’s four-step approach. For this purpose, after correspondence with the developer of the main version (Dr. James W. Varni) [25] and obtaining permission from him, the forward and backward translation was carried out. First, an Iranian translator and a nurse familiar with English translated the questionnaire from English to Persian. Then, an initial Persian draft was obtained after checking the quality of translations and making modifications. A nurse familiar with English back-translated it into English. To compare the translated English draft with the original version of the questionnaire, it was emailed to Dr. Varni. Finally, the final Persian draft was approved after revising and editing. The flowchart of the study process is presented in Figure 1.

Parents were asked to complete both parent proxy-report (for ages 2-18) and child self-report (for ages 2-4) versions. The versions for 5-18 years old were completed by children. The information for parents (including age, relationship with the child, education and economic status) and children (including age, sex, type of cancer, and disease status) were also recorded. During the completion of the questionnaires, the researcher was available and answered their questions. The average time to complete the questionnaires was 20 minutes.
For content validity assessment, 10 experts in the fields of oncology, pediatric nursing, and psychometrics were asked to give their opinions about the grammar, wording, item allocation, and compatibility of the final draft with Iranian culture. The feasibility and comprehensibility of the items were assessed by 10 parents of children with cancer.
To determine the construct validity, confirmatory factor analysis (CFA) was used. Maximum likelihood estimation was used to estimate the factor matrix. Several fit indices are used in CFA, including χ2/df, χ2, df, goodness of fit index (GFI), adjusted goodness of fit (AGFI), comparative fit index (CFI), root mean square residuals (RMSR), root mean square error of approximation (RMSEA), and normed fit index (NFI). The acceptable values were considered as χ2/df <5, CFI >0.9 , GFI >0.9, AGFI >0.9, and RMSEA <0.08 [26, 27]. Considering the studies assessed the different versions of the PedsQL 4.0, a sample size of 200 children and 200 parents was determined to perform the CFA. To determine the internal consistency of the Persian version, the questionnaire was given to 200 participants, and Cronbach’s α coefficient was calculated. To determine the test re-test reliability, the questionnaire was completed by 50 children aged 2-18 and their parents (n=50), and the Intraclass correlation coefficient (ICC) was calculated. Statistical analyses were done in SPSS software, version 21. Descriptive statistics (frequency, percentage, Mean±SD) were used to describe the data, to perform CFA, LISREL software, version 8.8 was used.
Results
The age of children and their parents was 5.9±4.2 and 37.83±5.86 years, respectively. Other demographic and clinical characteristics are presented in Table 1.

In the child self-report version, Cronbach’s α value for the eight subscales was in the range of 0.72-0.88 and for the overall scale, it was 0.80 (Table 2).

In the parent proxy-report version, Cronbach’s α value was in the range of 0.82-0.93 and for the overall scale, it was 0.81 (Table 3).

In determining the construct validity, all fit indices had acceptable value and confirmed the fitness of the final model (Table 4). Since the values were above 0.7 in both versions, it can be said that the Persian version had acceptable internal consistency.

For the test re-test reliability, the ICC values for each age group of children are presented in Table 5. Except for the treatment anxiety subscale in the 5-7 years age group and the worry subscale in the 13-18 years age group, the ICC values were good to excellent. The ICC values for the parent proxy-report version also were good to excellent .

Discussion
The present study investigated the psychometric properties of the Persian version of the PedsQL 4.0 for Iranian children with cancer. The results showed the appropriate reliability and validity of the Persian PedsQL 4.0. Construct validity in this research was done using the CFA. The main version of the PedsQL 4.0 cancer has eight factors [23]. Our results showed that the Persian version (for both child self-report and parent proxy-report) had a good fit to the data and the eight-factor model was confirmed.
According to the Cronbach’s α values, all subscales of the Persian PedsQL 4.0 in the child self-report and parent proxy-report versions for all age groups had good or acceptable internal consistency. In line with the present study, the results of a psychometric study on the Chinese version of the PedsQL™ 4.0 showed that the Cronbach’s α values for the whole scale and the subscales in the child self-report and parent proxy-report were at an acceptable level and had a good internal consistency [28]. For the Brazilian version, a study showed that the Cronbach’s α values for the child and parent versions were at a acceptable level, but in contrast to our study, most of the subscales were not at a good level [29]. For the original version, Cronbach’s α values for the subscales of “perceived physical appearance” and “communication” in the children self-report were not at acceptable level [23].
The results of test re-test reliability assessment showed that except for the “treatment anxiety” subscale for 5-7 year age group and the “worry” subscale for the 13-18 year age group, the ICC values for all subscales of child and parent versions and different age groups were good to excellent. For the Japanese version, the ICC values of the subscale “treatment anxiety” in children aged 5-7 and 13-18 years old and the subscale “worry” for children aged 8-12 years were in the low range [30]. For the Chinese version, the ICC values for all subscales of child and parent versions were in the good to excellent range [28]. In the Brazilian version, the ICC was not calculated separately for different age groups, but they were in the appropriate range for the whole scale [29]. The low ICC of the “treatment anxiety” subscale for 5-7 year age group in our study can be due to the difficulty of explaining treatment anxiety by the items in this subscale for young children. The low ICC of the “worry” subscale for children aged 13-18 years may be due to the difference in treatments during two weeks, according to the items in this subscale. In this study, data collection was done in one hospital in northern Iran, which can affect the generalizability of the results to other centers.
In conclusion, the Persian version of the PedsQL™ 4.0 has acceptable psychometric properties and can be used as a valid and reliable tool to assess the HRQOL of Iranian children with cancer. It can help researchers and physicians in Iran to better understand the experiences of children with cancer and create more effective interventions to improve their quality of life. It is recommended that future studies focus on the sensitivity and responsiveness of this tool and provide the results to compare data for healthy children.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1400.267). The participants were free to leave the study at any time and their information was kept confidential. Written informed consent was obtained from parents and verbal consent from children to participate in the study.
Funding
This study was extracted from a PhD dissertation of Somaye Pouy, approved by the Department of Nursing, Faculty of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. this study was financially supported by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
Conceptualization, design, and resources: Somaye Pouy, Zahra Taheri Ezbarami, Maryam Rassouli and Nazila Javadi-Pashaki; Data collection: Somaye Pouy, and Saman Maroufizadeh; Data analysis: Nazila Javadi-Pashaki, Somaye Pouy and Saman Maroufizadeh; Investigation, and writing the original draft: Somaye Pouy, Zahra Taheri Ezbarami, Maryam Rassouli and Nazila Javadi-Pashaki; Review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research and Technology of Guilan University of Medical Sciences and the officials and medical staff of the selected specialized hospitals for children for supporting this study, and all children and their parents who participated in this study for their cooperation.
References
- Chang WH, Katsoulis M, Tan YY, Mueller SH, Green K, Lai AG. Late effects of cancer in children, teenagers and young adults: Population-based study on the burden of 183 conditions, in-patient and critical care admissions and years of life lost. Lancet Reg Health Eur. 2021; 12:100248. [DOI:10.1016/j.lanepe.2021.100248] [PMID]
- Johnston WT, Erdmann F, Newton R, Steliarova-Foucher E, Schüz J, Roman E. Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 2021; 71(Pt B):101662. [DOI:10.1016/j.canep.2019.101662] [PMID]
- Lewandowska A, Zych B, Papp K, Zrubcová D, Kadučáková H, Šupínová M, et al. Problems, stressors and needs of children and adolescents with cancer. Children (Basel). 2021; 8(12):1173. [DOI:10.3390/children8121173] [PMID]
- WHO. CureAll framework: WHO global initiative for childhood cancer: increasing access, advancing quality, saving lives. Geneva: World Health Organization; 2021. [Link]
- Ahmadnia S, Ghalibaf AK, Kamkar S, Mohamadzadeh Z, Ghalibafian M. Survivor and parent engagement in childhood cancer treatment in Iran. Ecancermedicalscience. 2021; 15:1220. [DOI:10.3332/ecancer.2021.1220] [PMID]
- Guida F, Kidman R, Ferlay J, Schüz J, Soerjomataram I, Kithaka B, et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nat Med. 2022; 28(12):2563-72. [DOI:10.1038/s41591-022-02109-2] [PMID]
- Huang J, Chan SC, Ngai CH, Lok V, Zhang L, Lucero-Prisno 3rd DE, et al. Global incidence, mortality and temporal trends of cancer in children: A joinpoint regression analysis. Cancer Med. 2023; 12(2):1903-11. [DOI:10.1002/cam4.5009] [PMID]
- You L, Lv Z, Li C, Ye W, Zhou Y, Jin J, et al. Worldwide cancer statistics of adolescents and young adults in 2019: a systematic analysis of the Global Burden of Disease Study 2019. ESMO Open. 2021; 6(5):100255. [DOI:10.1016/j.esmoop.2021.100255] [PMID]
- Ferrández-Pujante A, Pérez-Silva A, Serna-Muñoz C, Fuster-Soler JL, Galera-Miñarro AM, Cabello I, et al. Prevention and treatment of oral complications in hematologic childhood cancer patients: an update. Children (Basel). 2022; 9(4):566. [DOI:10.3390/children9040566] [PMID]
- Malczewska M, Kośmider K, Bednarz K, Ostapińska K, Lejman M, Zawitkowska J. Recent advances in treatment options for childhood acute lymphoblastic leukemia. Cancers (Basel). 2022; 14(8):2021. [DOI:10.3390/cancers14082021] [PMID]
- Zahnreich S, Schmidberger H. Childhood cancer: occurrence, treatment and risk of second primary malignancies. Cancers (Basel). 2021; 13(11):2607. [DOI:10.3390/cancers13112607] [PMID]
- Bakker A, Streefkerka N, Bakker A, Gorp MV, Litsenburg RV, Grootenhuis M, et al. A systematic review of health-related quality of life in children and adolescents during treatment for cancer. EJC Paediatr Oncol. 2023. 2:100134. [DOI:10.1016/j.ejcped.2023.100134]
- Defar S, Abraham Y, Reta Y, Deribe B, Jisso M, Yeheyis T, et al. Health related quality of life among people with mental illness: The role of socio-clinical characteristics and level of functional disability. Front Public Health. 2023; 11:1134032. [DOI:10.3389/fpubh.2023.1134032] [PMID]
- Nunes MD, Jacob E, Lopes-Júnior LC, Leite AC, Lima RA, Nascimento LC. [Qualidade de vida da população infantojuvenil oncológica com e sem fadiga (Portuguese)]. Acta Paul Enferm. 2022; 35:eAPE0288345. [DOI:10.37689/acta-ape/2022AO0288345]
- Ehrhardt MJ, Krull KR, Bhakta N, Liu Q, Yasui Y, Robison LL, et al. Improving quality and quantity of life for childhood cancer survivors globally in the twenty-first century. Nat Rev Clin Oncol. 2023; 20(10):678-96. [DOI:10.1038/s41571-023-00802-w] [PMID]
- Hilgendorf I, Bergelt C, Bokemeyer C, Kaatsch P, Seifart U, Stein A, et al. Long-term follow-up of children, adolescents, and young adult cancer survivors. Oncol Res Treat. 2021; 44(4):184-9. [DOI:10.1159/000514381] [PMID]
- Fardell JE, Wakefield CE, De Abreu Lourenco R, Signorelli C, McCarthy M, McLoone J, et al. Long-term health-related quality of life in young childhood cancer survivors and their parents. Pediatr Blood Cancer. 2021; 68(12):e29398. [DOI:10.1002/pbc.29398] [PMID]
- Avoine-Blondin J, Dumont É, Marquis MA, Duval M, Sultan S. Quality of life in childhood advanced cancer: from conceptualization to assessment with the Advance QoL tool. BMC Palliat Care. 2022; 21(1):138. [DOI:10.1186/s12904-022-01025-z] [PMID]
- Ravens-Sieberer U, Erhart M, Wille N, Wetzel R, Nickel J, Bullinger M. Generic health-related quality-of-life assessment in children and adolescents: methodological considerations. Pharmacoeconomics. 2006; 24(12):1199-220. [DOI:10.2165/00019053-200624120-00005] [PMID]
- Varni JW, Limbers C, Burwinkle TM. Literature review: health-related quality of life measurement in pediatric oncology: hearing the voices of the children. J Pediatr Psychol. 2007; 32(9):1151-63. [DOI:10.1093/jpepsy/jsm008] [PMID]
- Bhatia S, Jenney ME, Bogue MK, Rockwood TH, Feusner JH, Friedman DL, et al. The Minneapolis-Manchester Quality of Life instrument: reliability and validity of the Adolescent Form. J Clin Oncol. 2002; 20(24):4692-8. [DOI:10.1200/JCO.2002.05.103] [PMID]
- Varni JW, Katz ER, Seid M, Quiggins DJ, Friedman-Bender A, Castro CM. The Pediatric Cancer Quality of Life Inventory (PCQL). I. Instrument development, descriptive statistics, and cross-informant variance. J Behav Med. 1998; 21(2):179-204. [DOI:10.1023/A:1018779908502] [PMID]
- Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002; 94(7):2090-106.[DOI:10.1002/cncr.10428] [PMID]
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999; 37(2):126-39. [DOI:10.1097/00005650-199902000-00003] [PMID]
- Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000; 25(24):3186-91. [DOI:10.1097/00007632-200012150-00014] [PMID]
- West SG, Taylor AB, Wu W. Model fit in structural equation modeling. In: Hoyle RH, editor. Handbook of structural equation modeling. New York: Guilford Publications; 2023. [Link]
- Steenkamp JBEM, Maydeu-Olivares A. Unrestricted factor analysis: A powerful alternative to confirmatory factor analysis. J Acad Mark Sci. 2023; 51:86-113. [DOI:10.1007/s11747-022-00888-1]
- Ji Y, Chen S, Li K, Xiao N, Yang X, Zheng S, et al. Measuring health-related quality of life in children with cancer living in Mainland China: feasibility, reliability and validity of the Chinese Mandarin version of PedsQL 4.0 Generic Core Scales and 3.0 Cancer Module. Health Qual Life Outcomes. 2011; 9:103. [DOI:10.1186/1477-7525-9-103] [PMID]
- Scarpelli AC, Paiva SM, Pordeus IA, Ramos-Jorge ML, Varni JW, Allison PJ. Measurement properties of the Brazilian version of the Pediatric Quality of Life Inventory (PedsQL) cancer module scale. Health Qual Life Outcomes. 2008; 6:7. [DOI:10.1186/1477-7525-6-7] [PMID]
- Tsuji N, Kakee N, Ishida Y, Asami K, Tabuchi K, Nakadate H, et al. Validation of the Japanese version of the Pediatric Quality of Life Inventory (PedsQL) Cancer Module. Health Qual Life Outcomes. 2011; 9:22. [DOI:10.1186/1477-7525-9-22] [PMID]
Article Type : Research |
Subject:
General
Received: 2024/05/22 | Accepted: 2025/02/23 | Published: 2025/04/1
Received: 2024/05/22 | Accepted: 2025/02/23 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



