Sat, Jan 31, 2026
Volume 35, Issue 1 (1-2025)
JHNM 2025, 35(1): 45-51 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aramesh S, Azaraksh F, Jannesar R, Iriti M, Bazarganipour F. Comparison of Vitamin D and Zinc Levels in Women With Diminished Ovarian Reserve Compared to Normal condition: A Case-control Study. JHNM 2025; 35 (1) :45-51
URL: http://hnmj.gums.ac.ir/article-1-2289-en.html
URL: http://hnmj.gums.ac.ir/article-1-2289-en.html
Shahintaj Aramesh1 

 , Fatemeh Azaraksh1
, Fatemeh Azaraksh1 

 , Ramin Jannesar2
, Ramin Jannesar2 

 , Marcello Iriti3
, Marcello Iriti3 

 , Fatemeh Bazarganipour *4
, Fatemeh Bazarganipour *4 




 , Fatemeh Azaraksh1
, Fatemeh Azaraksh1 

 , Ramin Jannesar2
, Ramin Jannesar2 

 , Marcello Iriti3
, Marcello Iriti3 

 , Fatemeh Bazarganipour *4
, Fatemeh Bazarganipour *4 


1- Associate Professor, Department of Gynecology and Obstetrics, School of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran.
2- Assistant Professor, Department of Pathology, School of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran.
3- Professor, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
4- Associate Professor, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran. ,f.bazarganipour@gmail.com
2- Assistant Professor, Department of Pathology, School of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran.
3- Professor, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy.
4- Associate Professor, Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran. ,
Full-Text [PDF 514 kb]
(423 Downloads)
| Abstract (HTML) (752 Views)
Full-Text: (505 Views)
Introduction
Ovarian reserve indicates ovarian functionality due to the quantity and quality of ovarian follicles [1]. In addition to the effects of genetics and age on ovarian reserve, environmental factors are thought to moderate the rate of ovarian reserve decline [1, 2]. It has been hypothesized that a possible effect of vitamin D (a steroid hormone that has known effects on calcium and bone metabolism [3]) on Anti-Müllerian hormone (AMH) may be a presumptive component explaining the complex association between vitamin D and human reproduction [4]. Kinuta et al. found that the neutral vitamin D receptor (VDR) in mice suffering from ovarian failure had impaired follicular growth [5]. A recent meta-analysis evaluated the fertility outcomes of 2,700 women with infertility and found a significant relationship between favorable outcomes and rich vitamin D status [6]. Based on a recent systematic review and meta-analysis of studies on vitamin D and AMH, more randomized controlled trials (RCTs) are needed to explain the complex relationship between vitamin D and AMH [7].
There is a feed-forward loop between zinc and vitamin D. Zinc can enhance vitamin D activities, while vitamin D can influence zinc homeostasis. Vitamin D functions are partly regulated by zinc finger-dependent transcription of vitamin D-dependent genes. When vitamin D binds to the VDR, it interacts with the zinc finger DNA-binding domain to regulate the transcriptional activation of genes to exert cellular functions. As mentioned, zinc is an essential cofactor to have the desired functions of vitamin D. Similarly, vitamin D can also influence zinc absorption and homeostasis by regulating its transporters [8]. Given the high prevalence of hypovitaminosis D and zinc deficiency [9, 10], there is a need to pay attention to their possible interactions in female reproduction. The role of zinc in women’s reproductive system is very important. An animal study showed that female rabbits with zinc deficiency could not ovulate. In addition, their endometrium was inactive, and they could not conceive [11]. A study on the zinc level in the follicular fluid of women undergoing in vitro fertilization reported a relationship between zinc concentration in the follicular fluid and follicle volume and the presence of oocytes in the follicle and oocyte fertilization [12].
To date, limited observational and interventional studies have been performed to evaluate the relationship between vitamin D and zinc serum levels and ovarian reserve, which have presented contradictory results. More studies are needed to investigate the relationship of diet and nutrition patterns with AMH concentration and age at menopause to better understand this issue. Therefore, this novel study aimed to assess the association of vitamin D and zinc dietary intake/serum levels with ovarian reserve in women with diminished ovarian reserve (DOR) in Yasuj, Iran.
Material and Methods
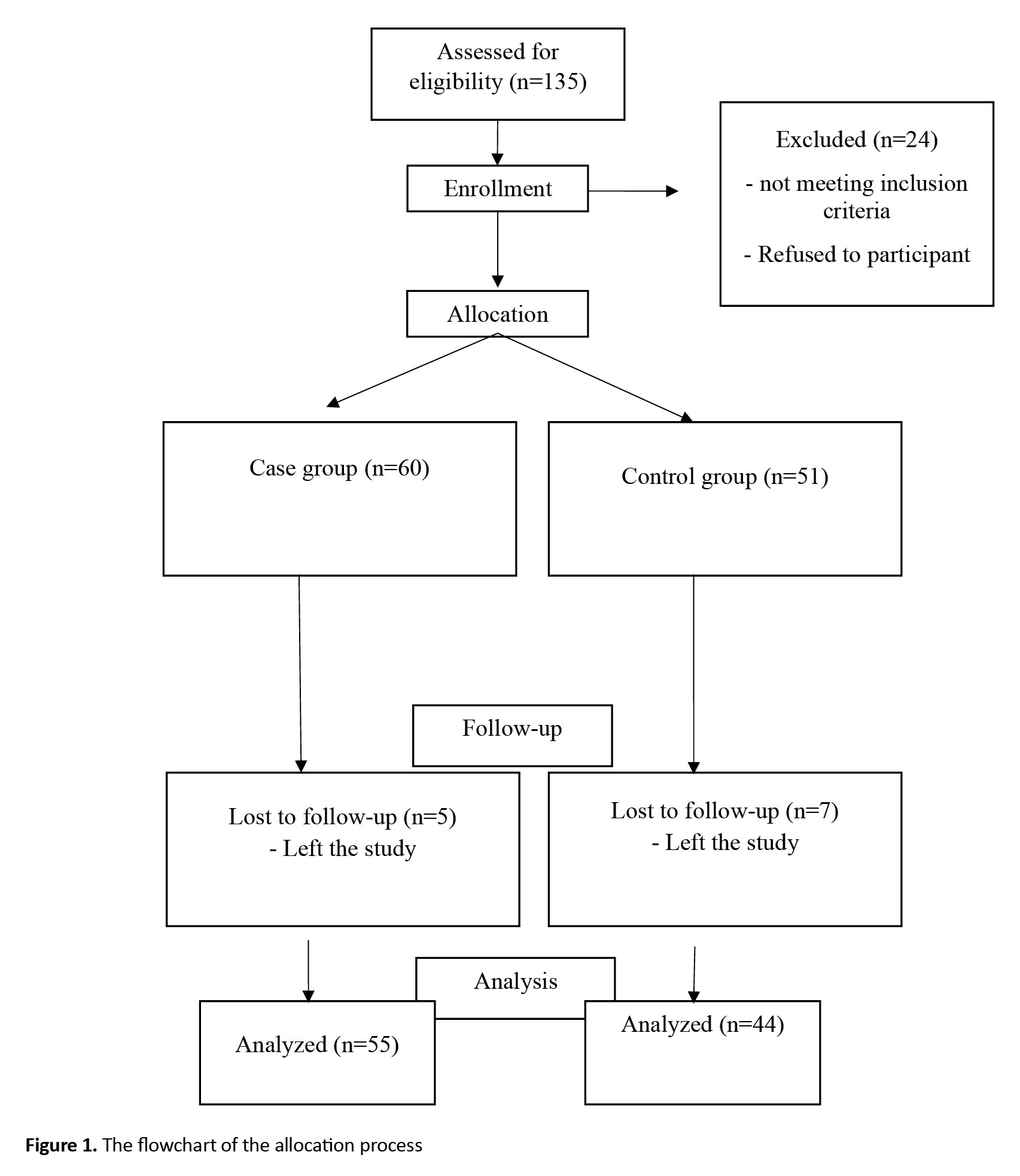
This is a case-control study that was conducted on women with infertility referred to two infertility treatment clinics in Yasuj, Iran, during 2019-2020. Considering the findings of Ersoy et al., including a median vitamin D level of 7.75 (ranged 3-21.22) in cases and 6.74 (ranged 3-25.54) in controls [13], α=0.05 and β=0.2, at least 40 people per group were considered as the sample size. A convenience sampling method was used to select participants. The inclusion criteria were willingness to contribute to the study, age 18-40 years, being married, having Iranian nationality, no history of systemic diseases (diabetes, hypertension, known anemia, autoimmune or inflammatory diseases, and other diseases needing special diet), no tobacco, alcohol, or drug use, no specific gynecological diseases (e.g. endometriosis, polycystic ovarian syndrome), no history of surgery in the uterus and ovaries, no history of receiving chemotherapy or radiotherapy, no hypercalcemia (plasma calcium concentrations >2.65 mmol/L), and no regular intake of vitamin D, zinc sulfate, or other supplements in the past three months based on medical records and self-reports. The exclusion criterion was the unwillingness to continue participation. Participants were divided into case and control groups. The case group included women with DOR (AMH level <1 ng/mL and antral follicle count (AFC) <10 in sonography at 3-10 days of menstrual cycle). The control group consisted of women with normal ovarian reserve. After presenting the purpose of the study to the participants, a written informed consent was obtained from them. The participants underwent transvaginal 2D ultrasound on day 2–5 of the menstrual cycle by an experienced clinician to confirm the AFC. Moreover, they underwent blood sampling for the assessment of their serum hormone profiles, zinc and vitamin D levels on days 2 to 5 of their menstrual cycle. The process of the allocation is shown in Figure 1. First, 135 patients were assessed for eligibility, of whom 24 were excluded because they did not meet the criteria. After allocation, 12 left the study (5 from the case group and 7 from the control group).
Demographic information, including age, occupation, and body mass index (BMI), were first recorded. To determine the serum levels of 25-hydroxyvitamin D, zinc, and AMH, the samples were first centrifuged and stored at -20 °C. The AMH assay was done with the ultra-sensitive AMH/MIS ELISA kit (Ansh Labs Co., USA) and an automated ELISA analyzer (Elisys Uno). Reproducibility and the total coefficient of variation for the AMH ELISA assay were 5.13, 6.03, and 4.46% at the concentrations of 0.35, 0.72, and 1.85 ng/mL, respectively. The serum level of zinc was measured by atomic absorption spectrometry (Chemtech analytical CTA-2000). The total imprecision coefficient of variation was 5.33% at a concentration level of 6.32 ng/mL, and 4.96% at the level of 37.71 ng/mL. The vitamin D assay was done by an ELISA kit (25-OH vitamin D kit, EuroImmun Co.) and an automated ELISA analyzer (Elisys Uno). The inter-assay precision was 7.8, 7, and 8.6% at the concentrations of 16.6, 43.5 and 67.8 ng/mL, respectively.
Dietary intake was measured using a modified food frequency questionnaire (FFQ) in Persian which had 168 items. The reliability and validity of the questionnaire have been approved in Iran [14]. The FFQ included a list of foods with response categories. Subjects were asked to report the frequency of consumption for each food in the past month on a daily, weekly or monthly basis. The frequency of consumption for each food item was converted to intake in grams per day using household units. This dietary information was analyzed in Nutrition software version 4, which calculated the amount of energy, macronutrients (carbohydrates, fats, and protein) and micronutrients (at least 30 micronutrients). We used only data related to dietary intake of vitamin D and zinc. This study was done in accordance with strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for observational studies [15].
The demographic data were expressed using Mean±SD, or percentage. The comparison of the data was performed by t-test or chi-square test. The effects of BMI and age were adjusted by the analysis of covariance. The statistical analyses were done in SPSS software, version 21 (SPSS, Chicago, IL) and P≤0.05 was considered as statistically significant. The normality of the data distribution was examined using the Kolmogorov-Smirnov test. There were no missing values. Therefore, no missing data imputation technique was used.
Results
The mean age of the participants was 33.4±26.58 years in the case group and 32.4±31.16 years in the control group. The mean BMI was 27.15±3.97 kg/m2 in the case group and 26.35±4.23 kg/m2 in the control group. Demographic and anthropometric characteristics of the patients are reported in Table 1.
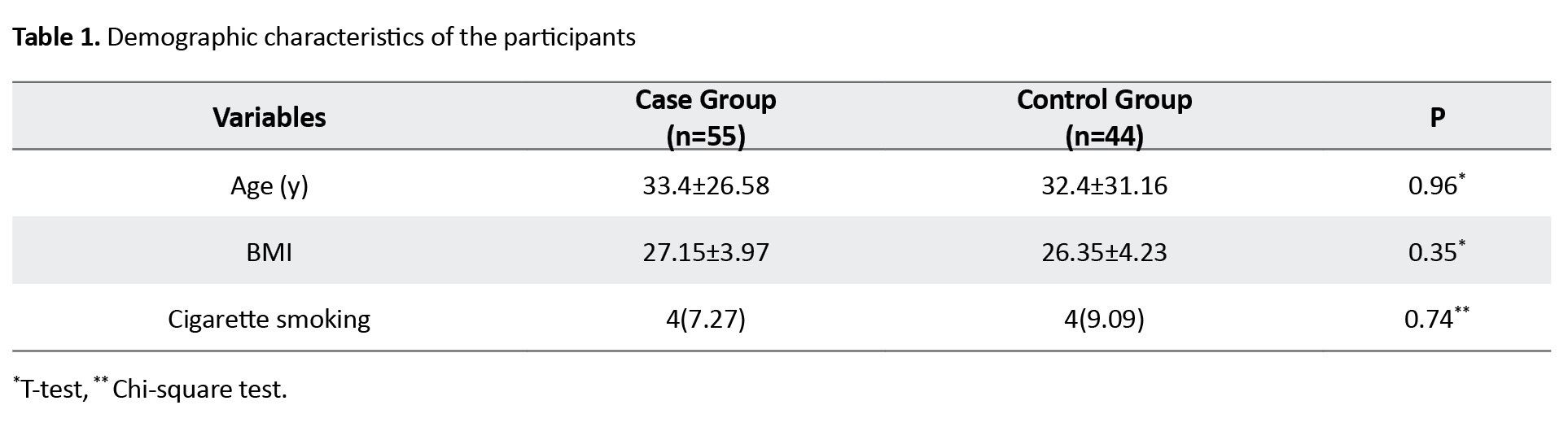
There was no significant difference between study groups in terms of these characteristics.
Table 2 shows the results of comparison between the two groups based on the dietary intake and serum level of vitamin D.
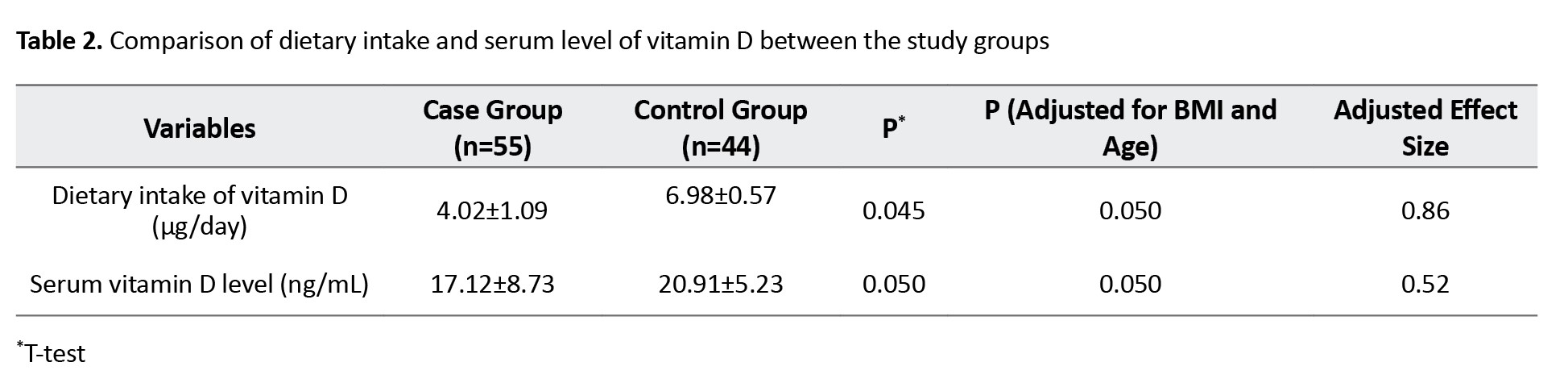
After adjustment of the effects of BMI and age, dietary intake (6.98±0.57 μg/day in the control group vs 4.02±1.09 μg/day in the case group, P=0.050) and serum vitamin D (20.91±5.23 ng/mL in the control group vs 17.12±8.73 ng/mL in the case group, P=0.050) levels were significantly higher in the control group compared to the case group. The adjusted effect size for age and BMI for dietary intake of vitamin D was 0.86; for the serum vitamin D level, it was 0.52.
Table 3 shows the results of comparison between the two groups based on the dietary intake and serum level of zinc.
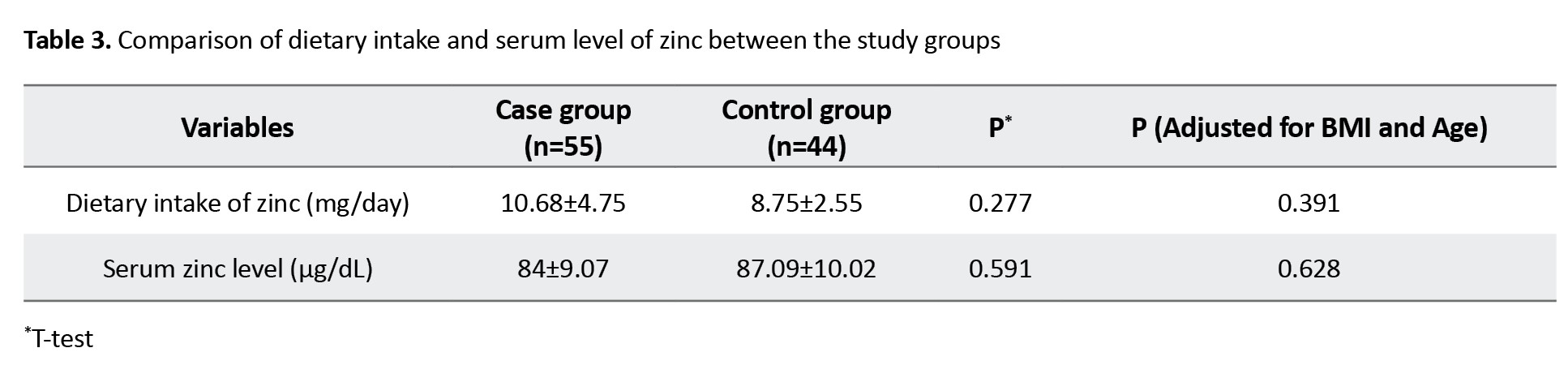
After adjustment of the effects of BMI and age, dietary intake of zinc (8.75±2.55 mg/day in the control group vs 10.68±4.75 mg/day in the case group; P=0.39) and serum zinc level (87.09±10.02 μg/dL in the control group vs 87.09±10.02 μg/dL in the case group; P=0.62) were not significantly different between the two groups.
Discussion
The results of the present study showed that, after adjusting for BMI and age, the vitamin D intake and serum vitamin D levels were significantly higher in the women with normal ovarian reserve compared to the women with DOR. The identification of VDRs in reproductive organs such as ovaries, endometrium, testes, hypothalamus and pituitary gland has shown the role of vitamin D in reproduction [4]. Our results are in agreement with the results of previous studies. Arefi et al. in a prospective observational study showed a significant negative correlation between age and vitamin D level and between age and AFC. Also, they reported a significant correlation between vitamin D level and AFC [16]. Merhi et al. found a positive correlation between serum vitamin D and AMH levels in patients with human immunodeficiency virus in late reproductive age. However, this association was not observed in women under 40 years of age [17]. Another prospective study examined the effect of vitamin D intake (oral dose of 5,000 units in the first week of the menstrual cycle) on the AMH level in 49 women of reproductive age with regular menstrual cycle. Their AMH level increased by 9.12% in one week compared to those in the placebo group, which was significant [18].
As previously introduced, vitamin D alters the AMH sensitivity in human granulosa cells by suppressing AMH type II receptor expression. Due to reduced sensitivity to AMH, more follicles reach final maturity and ovulation, thereby affecting ovarian storage [17]. In addition, the presence of vitamin D response element in the promoter region of AMH gene indicates that the level of vitamin D can also affect AMH production [19]. Based on these findings, Irani et al. suggested that vitamin D is a clinically useful marker for ovarian storage [4], although other studies have shown contradictory results. Shapiro et al. examined the relationship between vitamin D level and ovarian reserve parameters in women with infertility and reported that the vitamin D level was not associated with ovarian reserve [20]. Drakopoulos et al. in a prospective study reported that the vitamin D level was not associated with AFC [21]. For clarifying the role of vitamin D in ovarian reserve, further investigation on the correlation between serum vitamin D level and the markers of ovarian reserve, are needed particularly in women with DOR.
Zinc is the second most abundantly distributed trace element in the body. It is an abundant micronutrient in meat and seafood. It is a cofactor for more than 80 metalloenzymes involved in DNA transcription and protein synthesis. As DNA transcription is an important part of germ cell development, zinc plays an important role in reproduction. In addition, zinc finger protein is involved in the genetic expression of steroid hormone receptors, and has anti-apoptotic and antioxidant properties [22]. In our study we found no significant difference between women with normal ovarian reserve and in zinc intake and serum zinc level. Moreover, there was no significant correlation between the levels of serum zinc and the AFC and AMH. Kebapcilar et al examined zinc, copper, and vitamin D levels in patients with primary ovarian insufficiency and found that zinc level were significantly lower in these patients than in controls [23]. Differences in the mean age of participants, combinations of food items in diet, and covariates entered to the model as potential confounders can be the reasons for the discrepancy. Further studies with larger sample sizes are recommended to assess the relevance of zinc in patients with DOR. It should be noted that, in addition to the limited number of studies on the relationship between nutritional factors and ovarian reserve, existing studies are hampered by the use of inappropriate markers of ovarian reserve and small sample sizes. Compared to other markers of ovarian reserve, the follicle stimulating hormone is the most commonly used marker in the previous studies. However, the changes in the concentration of this hormone during the menstrual cycle and its changes due to unrelated ovarian conditions make it an inaccurate and insensitive marker for measuring ovarian reserve. Currently, AMH is suggested as a powerful marker for predicting ovarian reserve. Therefore, more studies measuring AMH or AFC are needed to understand the role of dietary intakes on ovarian reserve.
The main strengths of this study included the investigation of the intake of vitamin D and zinc using a validated tool (FFQ) and measuring the AMH, vitamin D and zinc serum levels in one day which can indicate the real status of both hormones, while in the majority of previous studies, stored blood samples are used [17, 18, 24]. Nevertheless, there were some limitations to the present study. Patients were selected from only two infertility treatment clinic in Yasuj, which limits the generalization of the results. Also, Iranian ethnicity differences were not assessed for vitamin D status. We did not measure the vitamin D binding protein level, as none of the previous studies assessed this protein. Instead, the 25-OH vitamin D as an accepted biomarker for vitamin D, was used.
In conclusion, our results on DOR revealed the possible favorable relationship of vitamin D intake and serum level of vitamin D with ovarian reserve performance, and this relationship is independent of age and BMI. Further studies, especially clinical trials, are recommended to clarify the potential positive effects of vitamin D on ovarian reserve in DOR women.
Ethical Considerations
Compliance with ethical guidelines
All procedures were in accordance with the ethical guidelines of the Helsinki Declaration and its later amendments. The study was approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran (Code: IR.YUMS.REC.1398.121). A written informed consent was obtained from all patients. All participants were free to leave the study at any time.
Funding
This research was funded by Yasuj University of Medical Sciences, Yasuj, Iran (Grant No.: 960240).
Authors' contributions
conceptualization, study design, writing, and final approval: All authors; Data collection: Shahintaj Aramesh and Fatemeh Azarakhsh; Data analysis: Ramin Jannesar; Supervision: Shahintaj Aramesh and Fatemeh Bazarganipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research of Yasuj University of Medical Sciences, Yasuj, Iran, for the financial support, and all participants for their cooperation in this research.
References
Ovarian reserve indicates ovarian functionality due to the quantity and quality of ovarian follicles [1]. In addition to the effects of genetics and age on ovarian reserve, environmental factors are thought to moderate the rate of ovarian reserve decline [1, 2]. It has been hypothesized that a possible effect of vitamin D (a steroid hormone that has known effects on calcium and bone metabolism [3]) on Anti-Müllerian hormone (AMH) may be a presumptive component explaining the complex association between vitamin D and human reproduction [4]. Kinuta et al. found that the neutral vitamin D receptor (VDR) in mice suffering from ovarian failure had impaired follicular growth [5]. A recent meta-analysis evaluated the fertility outcomes of 2,700 women with infertility and found a significant relationship between favorable outcomes and rich vitamin D status [6]. Based on a recent systematic review and meta-analysis of studies on vitamin D and AMH, more randomized controlled trials (RCTs) are needed to explain the complex relationship between vitamin D and AMH [7].
There is a feed-forward loop between zinc and vitamin D. Zinc can enhance vitamin D activities, while vitamin D can influence zinc homeostasis. Vitamin D functions are partly regulated by zinc finger-dependent transcription of vitamin D-dependent genes. When vitamin D binds to the VDR, it interacts with the zinc finger DNA-binding domain to regulate the transcriptional activation of genes to exert cellular functions. As mentioned, zinc is an essential cofactor to have the desired functions of vitamin D. Similarly, vitamin D can also influence zinc absorption and homeostasis by regulating its transporters [8]. Given the high prevalence of hypovitaminosis D and zinc deficiency [9, 10], there is a need to pay attention to their possible interactions in female reproduction. The role of zinc in women’s reproductive system is very important. An animal study showed that female rabbits with zinc deficiency could not ovulate. In addition, their endometrium was inactive, and they could not conceive [11]. A study on the zinc level in the follicular fluid of women undergoing in vitro fertilization reported a relationship between zinc concentration in the follicular fluid and follicle volume and the presence of oocytes in the follicle and oocyte fertilization [12].
To date, limited observational and interventional studies have been performed to evaluate the relationship between vitamin D and zinc serum levels and ovarian reserve, which have presented contradictory results. More studies are needed to investigate the relationship of diet and nutrition patterns with AMH concentration and age at menopause to better understand this issue. Therefore, this novel study aimed to assess the association of vitamin D and zinc dietary intake/serum levels with ovarian reserve in women with diminished ovarian reserve (DOR) in Yasuj, Iran.
Material and Methods
This is a case-control study that was conducted on women with infertility referred to two infertility treatment clinics in Yasuj, Iran, during 2019-2020. Considering the findings of Ersoy et al., including a median vitamin D level of 7.75 (ranged 3-21.22) in cases and 6.74 (ranged 3-25.54) in controls [13], α=0.05 and β=0.2, at least 40 people per group were considered as the sample size. A convenience sampling method was used to select participants. The inclusion criteria were willingness to contribute to the study, age 18-40 years, being married, having Iranian nationality, no history of systemic diseases (diabetes, hypertension, known anemia, autoimmune or inflammatory diseases, and other diseases needing special diet), no tobacco, alcohol, or drug use, no specific gynecological diseases (e.g. endometriosis, polycystic ovarian syndrome), no history of surgery in the uterus and ovaries, no history of receiving chemotherapy or radiotherapy, no hypercalcemia (plasma calcium concentrations >2.65 mmol/L), and no regular intake of vitamin D, zinc sulfate, or other supplements in the past three months based on medical records and self-reports. The exclusion criterion was the unwillingness to continue participation. Participants were divided into case and control groups. The case group included women with DOR (AMH level <1 ng/mL and antral follicle count (AFC) <10 in sonography at 3-10 days of menstrual cycle). The control group consisted of women with normal ovarian reserve. After presenting the purpose of the study to the participants, a written informed consent was obtained from them. The participants underwent transvaginal 2D ultrasound on day 2–5 of the menstrual cycle by an experienced clinician to confirm the AFC. Moreover, they underwent blood sampling for the assessment of their serum hormone profiles, zinc and vitamin D levels on days 2 to 5 of their menstrual cycle. The process of the allocation is shown in Figure 1. First, 135 patients were assessed for eligibility, of whom 24 were excluded because they did not meet the criteria. After allocation, 12 left the study (5 from the case group and 7 from the control group).
Demographic information, including age, occupation, and body mass index (BMI), were first recorded. To determine the serum levels of 25-hydroxyvitamin D, zinc, and AMH, the samples were first centrifuged and stored at -20 °C. The AMH assay was done with the ultra-sensitive AMH/MIS ELISA kit (Ansh Labs Co., USA) and an automated ELISA analyzer (Elisys Uno). Reproducibility and the total coefficient of variation for the AMH ELISA assay were 5.13, 6.03, and 4.46% at the concentrations of 0.35, 0.72, and 1.85 ng/mL, respectively. The serum level of zinc was measured by atomic absorption spectrometry (Chemtech analytical CTA-2000). The total imprecision coefficient of variation was 5.33% at a concentration level of 6.32 ng/mL, and 4.96% at the level of 37.71 ng/mL. The vitamin D assay was done by an ELISA kit (25-OH vitamin D kit, EuroImmun Co.) and an automated ELISA analyzer (Elisys Uno). The inter-assay precision was 7.8, 7, and 8.6% at the concentrations of 16.6, 43.5 and 67.8 ng/mL, respectively.
Dietary intake was measured using a modified food frequency questionnaire (FFQ) in Persian which had 168 items. The reliability and validity of the questionnaire have been approved in Iran [14]. The FFQ included a list of foods with response categories. Subjects were asked to report the frequency of consumption for each food in the past month on a daily, weekly or monthly basis. The frequency of consumption for each food item was converted to intake in grams per day using household units. This dietary information was analyzed in Nutrition software version 4, which calculated the amount of energy, macronutrients (carbohydrates, fats, and protein) and micronutrients (at least 30 micronutrients). We used only data related to dietary intake of vitamin D and zinc. This study was done in accordance with strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for observational studies [15].
The demographic data were expressed using Mean±SD, or percentage. The comparison of the data was performed by t-test or chi-square test. The effects of BMI and age were adjusted by the analysis of covariance. The statistical analyses were done in SPSS software, version 21 (SPSS, Chicago, IL) and P≤0.05 was considered as statistically significant. The normality of the data distribution was examined using the Kolmogorov-Smirnov test. There were no missing values. Therefore, no missing data imputation technique was used.
Results
The mean age of the participants was 33.4±26.58 years in the case group and 32.4±31.16 years in the control group. The mean BMI was 27.15±3.97 kg/m2 in the case group and 26.35±4.23 kg/m2 in the control group. Demographic and anthropometric characteristics of the patients are reported in Table 1.

There was no significant difference between study groups in terms of these characteristics.
Table 2 shows the results of comparison between the two groups based on the dietary intake and serum level of vitamin D.

After adjustment of the effects of BMI and age, dietary intake (6.98±0.57 μg/day in the control group vs 4.02±1.09 μg/day in the case group, P=0.050) and serum vitamin D (20.91±5.23 ng/mL in the control group vs 17.12±8.73 ng/mL in the case group, P=0.050) levels were significantly higher in the control group compared to the case group. The adjusted effect size for age and BMI for dietary intake of vitamin D was 0.86; for the serum vitamin D level, it was 0.52.
Table 3 shows the results of comparison between the two groups based on the dietary intake and serum level of zinc.

After adjustment of the effects of BMI and age, dietary intake of zinc (8.75±2.55 mg/day in the control group vs 10.68±4.75 mg/day in the case group; P=0.39) and serum zinc level (87.09±10.02 μg/dL in the control group vs 87.09±10.02 μg/dL in the case group; P=0.62) were not significantly different between the two groups.
Discussion
The results of the present study showed that, after adjusting for BMI and age, the vitamin D intake and serum vitamin D levels were significantly higher in the women with normal ovarian reserve compared to the women with DOR. The identification of VDRs in reproductive organs such as ovaries, endometrium, testes, hypothalamus and pituitary gland has shown the role of vitamin D in reproduction [4]. Our results are in agreement with the results of previous studies. Arefi et al. in a prospective observational study showed a significant negative correlation between age and vitamin D level and between age and AFC. Also, they reported a significant correlation between vitamin D level and AFC [16]. Merhi et al. found a positive correlation between serum vitamin D and AMH levels in patients with human immunodeficiency virus in late reproductive age. However, this association was not observed in women under 40 years of age [17]. Another prospective study examined the effect of vitamin D intake (oral dose of 5,000 units in the first week of the menstrual cycle) on the AMH level in 49 women of reproductive age with regular menstrual cycle. Their AMH level increased by 9.12% in one week compared to those in the placebo group, which was significant [18].
As previously introduced, vitamin D alters the AMH sensitivity in human granulosa cells by suppressing AMH type II receptor expression. Due to reduced sensitivity to AMH, more follicles reach final maturity and ovulation, thereby affecting ovarian storage [17]. In addition, the presence of vitamin D response element in the promoter region of AMH gene indicates that the level of vitamin D can also affect AMH production [19]. Based on these findings, Irani et al. suggested that vitamin D is a clinically useful marker for ovarian storage [4], although other studies have shown contradictory results. Shapiro et al. examined the relationship between vitamin D level and ovarian reserve parameters in women with infertility and reported that the vitamin D level was not associated with ovarian reserve [20]. Drakopoulos et al. in a prospective study reported that the vitamin D level was not associated with AFC [21]. For clarifying the role of vitamin D in ovarian reserve, further investigation on the correlation between serum vitamin D level and the markers of ovarian reserve, are needed particularly in women with DOR.
Zinc is the second most abundantly distributed trace element in the body. It is an abundant micronutrient in meat and seafood. It is a cofactor for more than 80 metalloenzymes involved in DNA transcription and protein synthesis. As DNA transcription is an important part of germ cell development, zinc plays an important role in reproduction. In addition, zinc finger protein is involved in the genetic expression of steroid hormone receptors, and has anti-apoptotic and antioxidant properties [22]. In our study we found no significant difference between women with normal ovarian reserve and in zinc intake and serum zinc level. Moreover, there was no significant correlation between the levels of serum zinc and the AFC and AMH. Kebapcilar et al examined zinc, copper, and vitamin D levels in patients with primary ovarian insufficiency and found that zinc level were significantly lower in these patients than in controls [23]. Differences in the mean age of participants, combinations of food items in diet, and covariates entered to the model as potential confounders can be the reasons for the discrepancy. Further studies with larger sample sizes are recommended to assess the relevance of zinc in patients with DOR. It should be noted that, in addition to the limited number of studies on the relationship between nutritional factors and ovarian reserve, existing studies are hampered by the use of inappropriate markers of ovarian reserve and small sample sizes. Compared to other markers of ovarian reserve, the follicle stimulating hormone is the most commonly used marker in the previous studies. However, the changes in the concentration of this hormone during the menstrual cycle and its changes due to unrelated ovarian conditions make it an inaccurate and insensitive marker for measuring ovarian reserve. Currently, AMH is suggested as a powerful marker for predicting ovarian reserve. Therefore, more studies measuring AMH or AFC are needed to understand the role of dietary intakes on ovarian reserve.
The main strengths of this study included the investigation of the intake of vitamin D and zinc using a validated tool (FFQ) and measuring the AMH, vitamin D and zinc serum levels in one day which can indicate the real status of both hormones, while in the majority of previous studies, stored blood samples are used [17, 18, 24]. Nevertheless, there were some limitations to the present study. Patients were selected from only two infertility treatment clinic in Yasuj, which limits the generalization of the results. Also, Iranian ethnicity differences were not assessed for vitamin D status. We did not measure the vitamin D binding protein level, as none of the previous studies assessed this protein. Instead, the 25-OH vitamin D as an accepted biomarker for vitamin D, was used.
In conclusion, our results on DOR revealed the possible favorable relationship of vitamin D intake and serum level of vitamin D with ovarian reserve performance, and this relationship is independent of age and BMI. Further studies, especially clinical trials, are recommended to clarify the potential positive effects of vitamin D on ovarian reserve in DOR women.
Ethical Considerations
Compliance with ethical guidelines
All procedures were in accordance with the ethical guidelines of the Helsinki Declaration and its later amendments. The study was approved by the Ethics Committee of Yasuj University of Medical Sciences, Yasuj, Iran (Code: IR.YUMS.REC.1398.121). A written informed consent was obtained from all patients. All participants were free to leave the study at any time.
Funding
This research was funded by Yasuj University of Medical Sciences, Yasuj, Iran (Grant No.: 960240).
Authors' contributions
conceptualization, study design, writing, and final approval: All authors; Data collection: Shahintaj Aramesh and Fatemeh Azarakhsh; Data analysis: Ramin Jannesar; Supervision: Shahintaj Aramesh and Fatemeh Bazarganipour.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy for Research of Yasuj University of Medical Sciences, Yasuj, Iran, for the financial support, and all participants for their cooperation in this research.
References
- Amanvermez R, Tosun M. An update on ovarian aging and ovarian reserve tests. Int J Fertil Steril. 2016; 9(4):411-5. [PMID]
- Pelosi E, Simonsick E, Forabosco A, Garcia-Ortiz JE, Schlessinger D. Dynamics of the ovarian reserve and impact of genetic and epidemiological factors on age of menopause. Biol Reprod. 2015; 92(5):130. [DOI:10.1095/biolreprod.114.127381] [PMID]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357(3):266-81. [DOI:10.1056/NEJMra070553] [PMID]
- Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil Steril. 2014; 102(2):460-8.e3. [DOI:10.1016/j.fertnstert.2014.04.046] [PMID]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000; ;141(4):1317-24. [DOI:10.1210/endo.141.4.7403] [PMID]
- Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum Reprod. 2018; 33(1):65-80. [DOI:10.1093/humrep/dex326] [PMID]
- Moridi I, Chen A, Tal O, Tal R. The association between Vitamin D and Anti-Müllerian Hormone: A systematic review and meta-analysis. Nutrients. 2020; 12(6):1567. [DOI:10.3390/nu12061567] [PMID]
- Amos A, Razzaque MS. Zinc and its role in vitamin D function. Curr Res Physiol. 2022; 5:203-7. [DOI:10.1016/j.crphys.2022.04.001] [PMID]
- Tabrizi R, Moosazadeh M, Akbari M, Dabbaghmanesh MH, Mohamadkhani M, Asemi Z, et al. High prevalence of vitamin D Deficiency among Iranian Population: A systematic review and meta-analysis. Iran J Med Sci. 2018; 43(2):125-39. [PMID]
- Eslami MJ, Khoshhali M, Kelishadi R. A systematic review and meta-analysis on the prevalence of zinc deficiency in Iranian population. J Pediatr Rev. 2023; 11(3):209-20. [DOI:10.32598/jpr.11.3.451.1]
- Choi S, Liu X, Pan Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol Sin. 2018; 39(7):1120-32. [DOI:10.1038/aps.2018.25] [PMID]
- Janati S, Behmanesh MA, Najafzadehvarzi H, Akhundzade Z, Poormoosavi SM. Follicular fluid zinc level and oocyte maturity and embryo quality in women with polycystic ovary syndrome. Int J Fertil Steril. 2021; 15(3):197-201. [PMID]
- Ersoy E, Ersoy AO, Yildirim G, Buyukkagnici U, Tokmak A, Yilmaz N. Vitamin D levels in patients with premature ovarian failure. Ginekol Pol. 2016; 87(1):32-6. [DOI:10.17772/gp/57839] [PMID]
- Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency Questionnaire Developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010; 20(2):150-8. [DOI:10.2188/jea.JE20090083] [PMID]
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019; 13(Suppl 1):S31-4. [DOI:10.4103/sja.SJA_543_18] [PMID]
- Arefi S, Khalili G, Iranmanesh H, Farifteh F, Hosseini A, Fatemi HM, et al. Is the ovarian reserve influenced by vitamin D deficiency and the dress code in an infertile Iranian population? J Ovarian Res. 2018; 11(1):62. [DOI:10.1186/s13048-018-0435-7] [PMID]
- Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil Steril. 2012; 98(1):228-34. [DOI:10.1016/j.fertnstert.2012.03.029] [PMID]
- Dennis NA, Houghton LA, Pankhurst MW, Harper MJ, McLennan IS. Acute supplementation with high dose vitamin D3 increases serum anti-Müllerian hormone in young women. Nutrients. 2017; 9(7):719. [DOI:10.3390/nu9070719] [PMID]
- Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: Regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009; 150(4):1580-7. [DOI:10.1210/en.2008-1555] [PMID]
- Shapiro AJ, Darmon SK, Barad DH, Gleicher N, Kushnir VA. Vitamin D levels are not associated with ovarian reserve in a group of infertile women with a high prevalance of diminished ovarian reserve. Fertil Steril. 2018; 110(4):761-6.e1. [DOI:10.1016/j.fertnstert.2018.05.005] [PMID]
- Drakopoulos P, van de Vijver A, Schutyser V, Milatovic S, Anckaert E, Schiettecatte J, et al. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum Reprod. 2017; 32(1):208-14. [DOI:10.1093/humrep/dew304]
- Vickram S, Rohini K, Srinivasan S, Nancy Veenakumari D, Archana K, Anbarasu K, et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int J Mol Sci. 2021; 22(4):2188. [DOI:10.3390/ijms22042188] [PMID]
- Kebapcilar AG, Kulaksizoglu M, Kebapcilar L, Gonen MS, Unlü A, Topcu A, et al. Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper? Menopause. 2013; 20(1):94-9. [DOI:10.1097/gme.0b013e31826015ca] [PMID]
- Pearce K, Gleeson K, Tremellen K. Serum anti-Mullerian hormone production is not correlated with seasonal fluctuations of vitamin D status in ovulatory or PCOS women. Hum Reprod. 2015; 30(9):2171-7. [DOI:10.1093/humrep/dev167] [PMID]
Article Type : Research |
Subject:
Special
Received: 2024/02/3 | Accepted: 2024/04/29 | Published: 2025/01/12
Received: 2024/02/3 | Accepted: 2024/04/29 | Published: 2025/01/12
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |