Fri, Jan 30, 2026
Volume 35, Issue 1 (1-2025)
JHNM 2025, 35(1): 52-61 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jalali H, Rastkar Mehrabani E, Shirinabadi Farahani A, Nasiri M, Roosta Z, Varzeshnejad M. The Incidence Rate of Central Line-associated Blood Stream Infections Related to Peripherally Inserted Central Catheters and Its Related Factors in the Neonatal Intensive Care Units. JHNM 2025; 35 (1) :52-61
URL: http://hnmj.gums.ac.ir/article-1-2273-en.html
URL: http://hnmj.gums.ac.ir/article-1-2273-en.html
Hamide Jalali1 

 , Elahe Rastkar Mehrabani2
, Elahe Rastkar Mehrabani2 

 , Azam Shirinabadi Farahani3
, Azam Shirinabadi Farahani3 

 , Malihe Nasiri4
, Malihe Nasiri4 

 , Zahra Roosta5
, Zahra Roosta5 

 , Maryam Varzeshnejad *6
, Maryam Varzeshnejad *6 




 , Elahe Rastkar Mehrabani2
, Elahe Rastkar Mehrabani2 

 , Azam Shirinabadi Farahani3
, Azam Shirinabadi Farahani3 

 , Malihe Nasiri4
, Malihe Nasiri4 

 , Zahra Roosta5
, Zahra Roosta5 

 , Maryam Varzeshnejad *6
, Maryam Varzeshnejad *6 


1- MSc Student in Nursing, Student Research Committee, Department of Pediatric Nursing, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Nursing (MSc), Nursing Clinical Research Development Center, Mahdiyeh Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Associate Professor of Nursing, Department of Pediatric Nursing, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Assistant Professor, Department of Basic Sciences, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences. Tehran, Iran.
5- Clinical Research Development Center, Mahdiyeh Educational hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Nursing (Bs), Clinical Research Development Center, Mahdiyeh Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,m.varzeshnejad@gmail.com
2- Nursing (MSc), Nursing Clinical Research Development Center, Mahdiyeh Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Associate Professor of Nursing, Department of Pediatric Nursing, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Assistant Professor, Department of Basic Sciences, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences. Tehran, Iran.
5- Clinical Research Development Center, Mahdiyeh Educational hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
6- Nursing (Bs), Clinical Research Development Center, Mahdiyeh Educational Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
Keywords: Central line-associated blood bloodstream infections, Peripherally inserted central catheters (PICCs), Neonatal intensive care units (NICUs), Infants
Full-Text [PDF 494 kb]
(444 Downloads)
| Abstract (HTML) (865 Views)
Full-Text: (581 Views)
Introduction
The neonatal period, the first 28 days of life, is the most vulnerable time for an individual’s survival [1]. Although the mortality rate in the neonatal period is nearly 30 times higher than in later stages of life [2], advancements in neonatology science have led to unprecedented improvements in neonatal survival [3]. With the increase in the survival of new-borns, the importance of vascular access in new-borns has also increased [4]. The use of peripheral vascular catheters is a common and invasive procedure in new-borns and is one of the most frequently performed painful procedures in neonatal care [5]. Routine vein access methods can have complications [6]. Repeated attempts to access peripheral veins can compromise the delicate skin of new-borns (which serves as the first line of defence against systemic infections) and puts the infant at risk of further injury [7].
The placement of a peripherally inserted central catheter (PICC) is a safe, convenient, and effective vascular access method due to its simplicity, high success rate, low complications, and the ability to administer hyperosmolar substances. It can reduce the excessive painful stimuli caused by repeated venepunctures in babies, ensuring timely intravenous nutrition and medication administration. However, the use of PICCs is associated with the risk of central line-associated bloodstream infection (CLABSI). These complications may even more occur in new-borns with severe illnesses [8]. The rate of CLABSI in new-borns is in a range of 0.3-8.21 per 1000 catheter/days. The CLABSI is among the most significant causes of late-onset sepsis [9]. Therefore, preventing CLABSIs resulted from central venous catheterization is a top priority in neonatal care units, as it can reduce mortality, morbidity, and the length of hospital stays.
Several studies have attempted to identify the causes and factors associated with CLABSI in new-borns with PICCs, but these factors have not been definitively established yet [4, 10, 11]. Various variables such as weight, gender, gestational age [11], catheter dwell time, site of insertion, and the number of tries for catheter insertion have been introduced as potential factors associated with catheter-related infections [4]. The type of catheter, catheter tip position, gestational age, and birth weight are other risk factors, although there is no clear consensus on which factor truly affects the occurrence of this infection [12]. Trained nurses are responsible for PICC placement in clinical settings [13]. In the nursing interventions classification (NIC) system, PICC placement and care are considered a standardized nursing intervention [14]. This research aims to investigate the rate of CLABSI related to PICC in the neonatal intensive care units (NICUs) of hospitals in Tehran, Iran, and identify the associated factors.
Materials and Methods
This is a retrospective analytical study with a cross-sectional design. The medical records of all infants with PICC admitted to two NICUs of one maternal and neonatal hospital in Tehran, Iran, over 5 years (2018-2023) were assessed, and the data of 321 cases that met the inclusion criteria were extracted. The inclusion criteria were having a PICC during hospitalization for more than 24 hours. The infants with catheters transferred to other hospitals for continued treatment (catheter removal in another hospital) were excluded.
For data collection, a demographic/clinical checklist was designed after identifying all possible factors associated with PICC-related infection from previous studies. The researcher-made checklist included three sections: a) Demographic/clinical information related to the infant (sex, birth weight, gestational age, cause of hospitalization, weight on the day of PICC insertion, post-natal age on the day of catheter insertion, weight on the day of PICC removal, age on the day of full enteral feeding, total days of hospitalization, days of mechanical ventilation at the catheter dwell time, days of antibiotic therapy days at the catheter dwell time); b) Information related to the catheter (catheter size, neonatal age at the catheterization time, number of catheterization attempts, site of insertion, location of catheter tip after insertion, dwell time, cause of catheter removal, and type of catheter-related complications), and c) Information related to CLABSI, including laboratory test results. The validity of the checklist was confirmed based on a content validity ratio of 0.87 and a content validity index of 0.88, according to the opinions of 10 nursing faculty members. The Mann-Whitney U test and the logistic regression analysis were used for data analysis in SPSS software, version 20.
Results
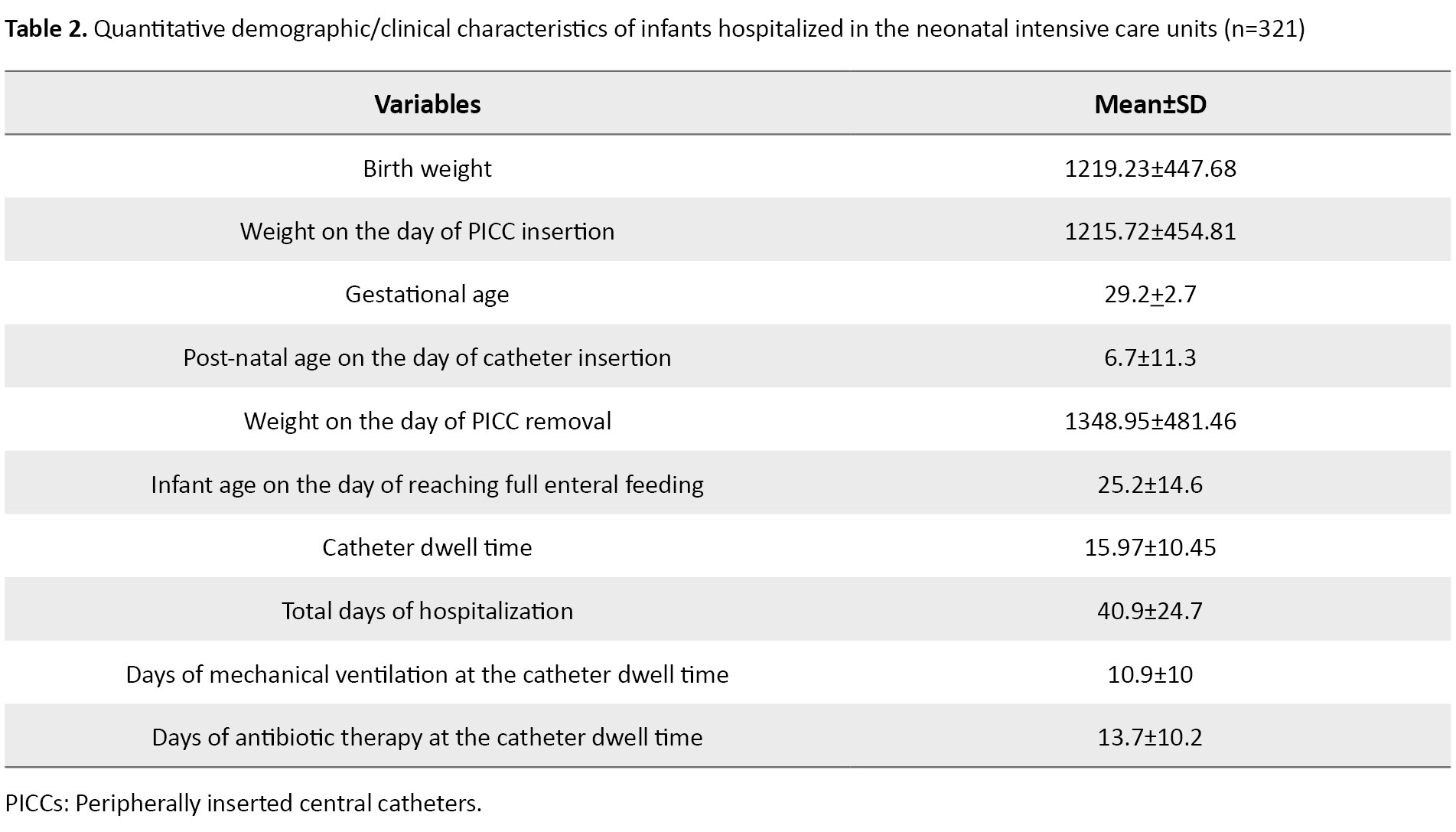
Of 321 infants with PICC, 168(52.3%) were male and 153(47.6%) were female. Their mean birth weight was 1219.23±447.68 g, while their mean weight on the day of PICC insertion was 1215.72±454.81 g, and the mean weight on the day of PICC removal was 1348.95±481.46 g, which was not significantly different from the weight on the day of PICC insertion. These results indicate that the insertion of PICC was mostly done for infants with weight <1500 g, and the infants had weight <1500 g until the time of PICC removal. The mean gestational age of the infants was 29.2±2.7 weeks, indicating that most of the infants were preterm. The mean post-natal age of the infants on the day of catheter insertion was 6.79±11.38. It also showed that most infants had catheter insertion in the first week of life.
The mean catheter dwell time was 15.97±10.45 days, and the total days of hospitalization was 40.9±24.7. The most common clinical diagnosis for infants was sepsis (49.53%). None of the infants had the clinical diagnosis of CLABSI. For 84.1% of the infants, a size 1 catheter had been inserted. Moreover, 62.3% of the infants had their PICC inserted at the first attempt. In 77.8% of cases, the catheter was placed in the upper extremities. Additionally, 85% of infants were under mechanical ventilation. The most common cause of catheter removal was “complications” (about 40%). In most cases, the catheter insertion was performed by a PICC team member (88.7%). Almost all infants (98.7%) were under antibiotic therapy at the time of catheter insertion. The majority of infants (25.86%) had an Apgar score of 9 at 1 minute, and 37% of infants had an Apgar score of 10 at 5 minutes. Other information is presented in Tables 1 and 2.
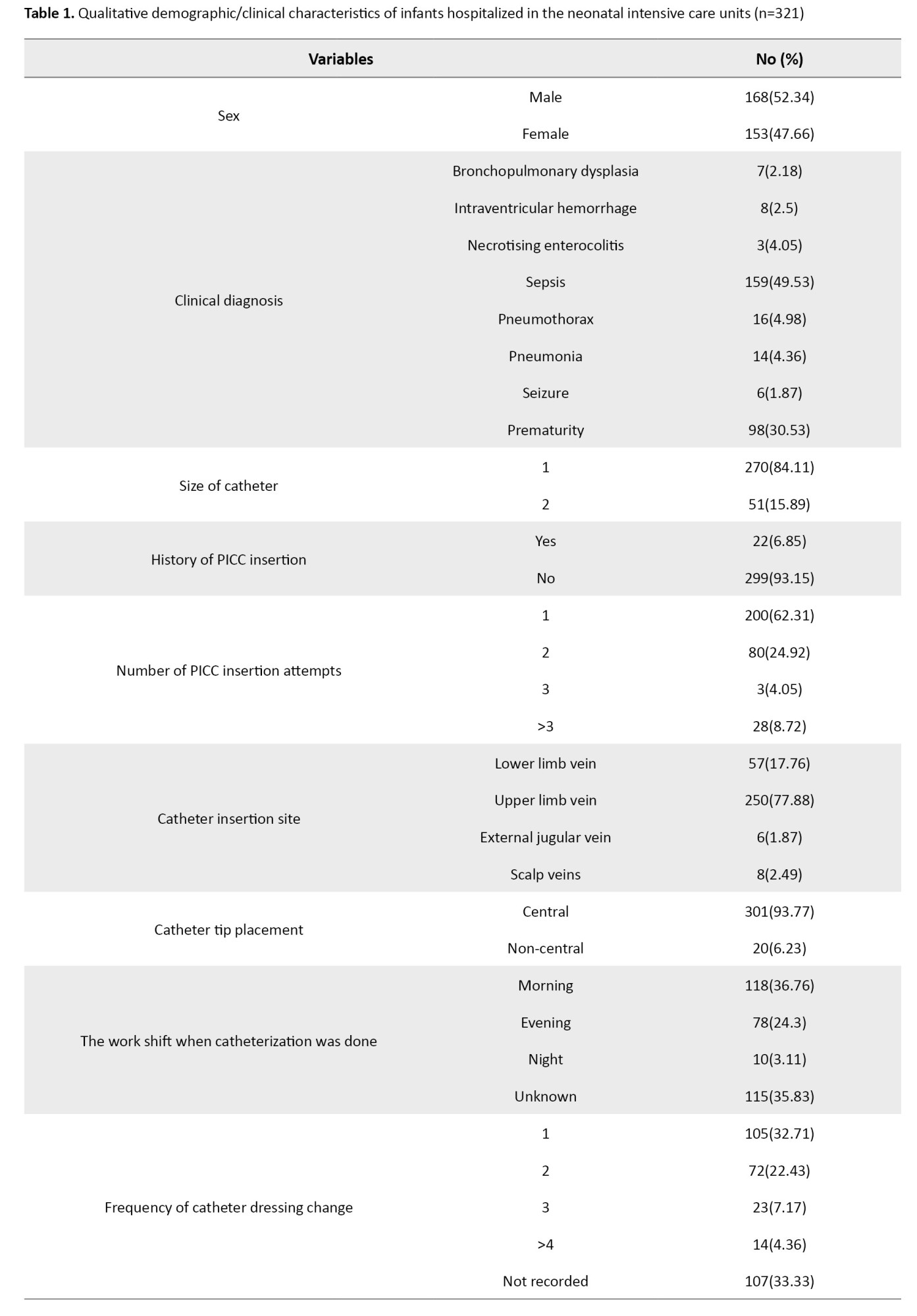
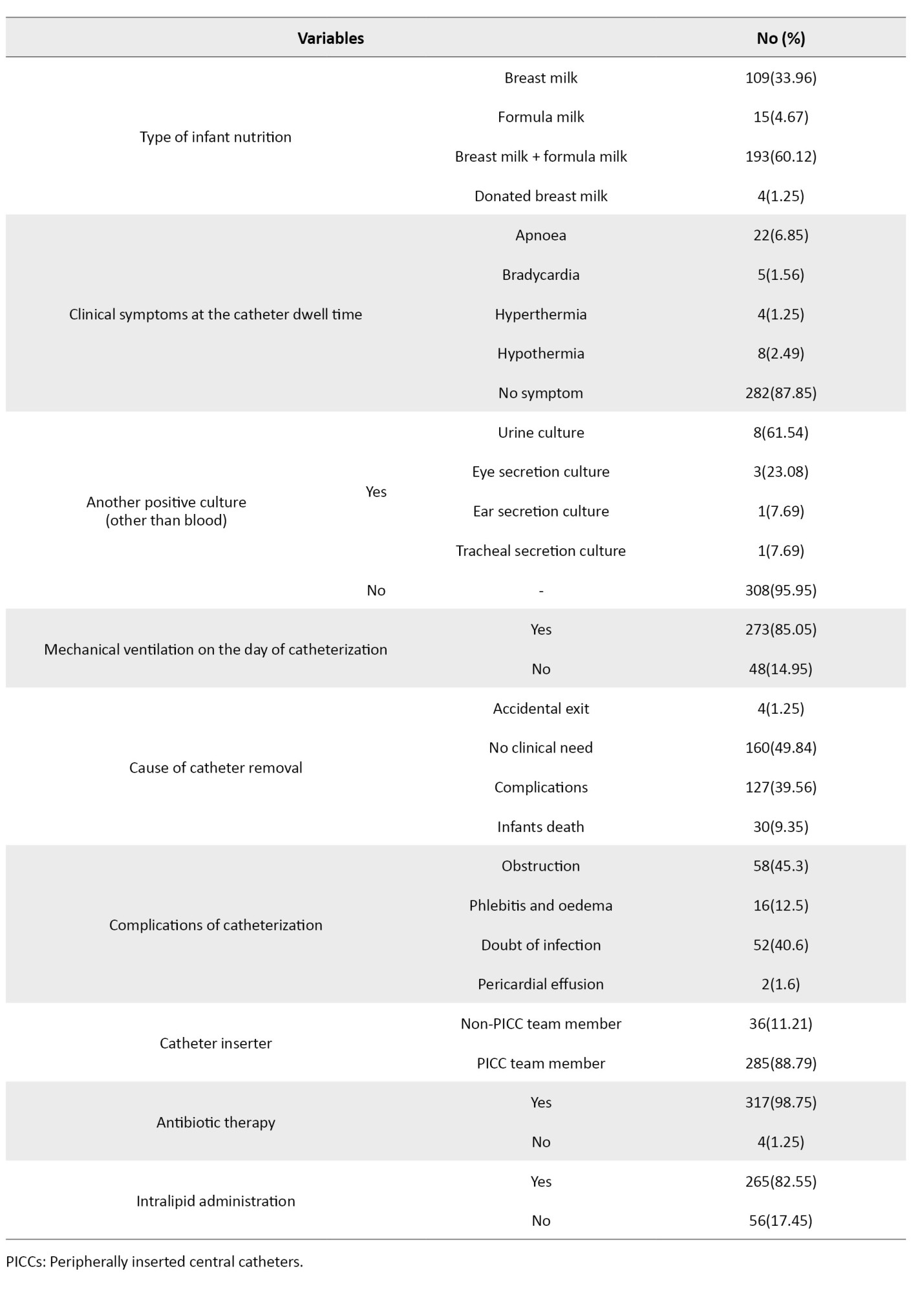
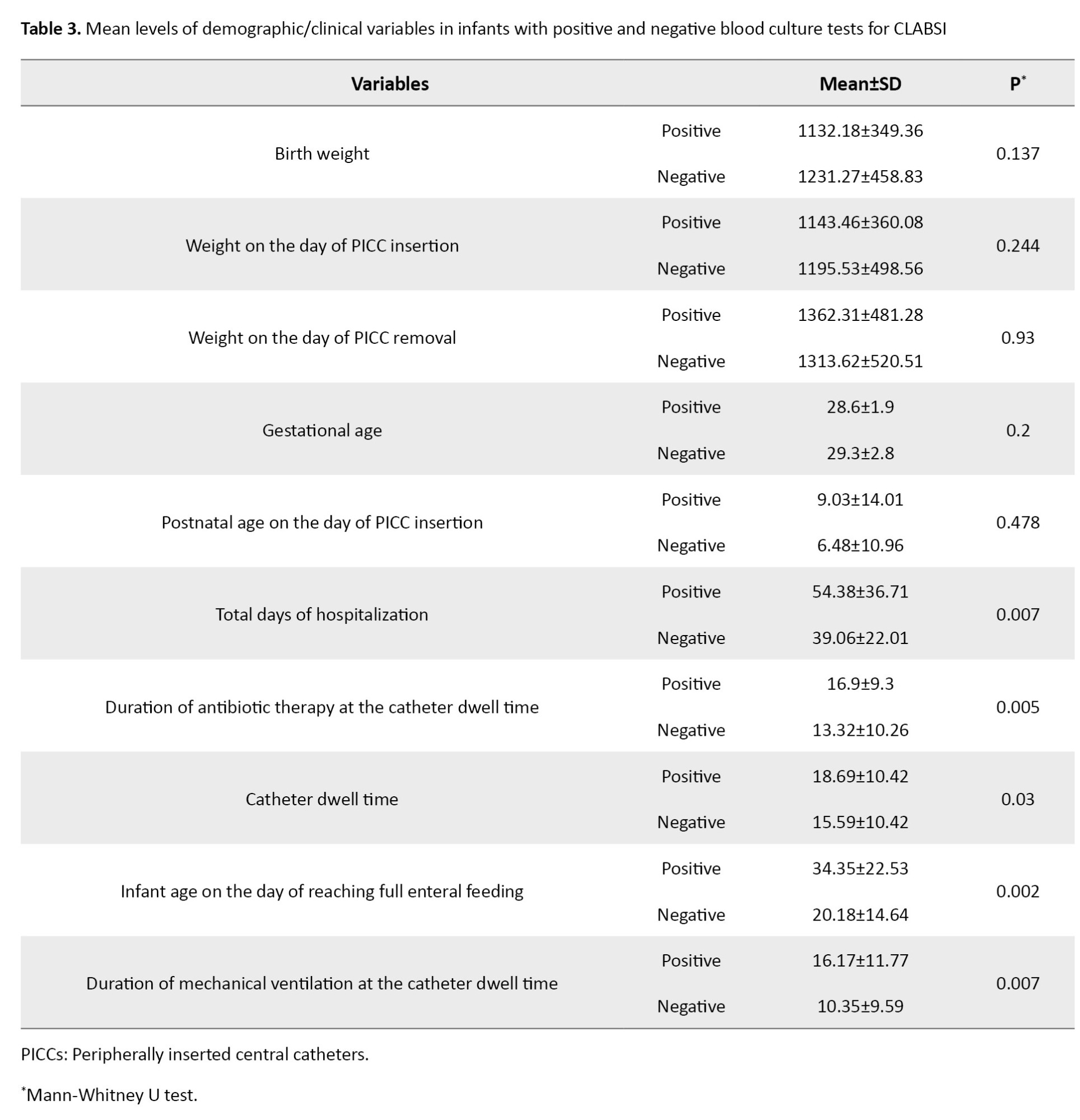
The rate of CLABSI caused by PICC was 7.60 per 1000 catheters/days. The Mann-Whitney U test results showed statistically significant differences between infants with positive blood culture test for CLABS and those with negative blood cultures in terms of total days of hospitalization (P=0.007), antibiotics therapy at the catheter dwell time (P=0.005), infant age on the day of reaching full enteral feeding (P=0.002), mechanical ventilation at the catheter dwell time (P=0.007), cause of catheter removal (P=0.001), previous history of PICC insertion (P=0.003), catheter dwell time (P=0.03) and duration of antibiotic therapy (P=0.005). In the rest of the variables, no significant differences were found between infants with positive and negative blood cultures (Table 3).
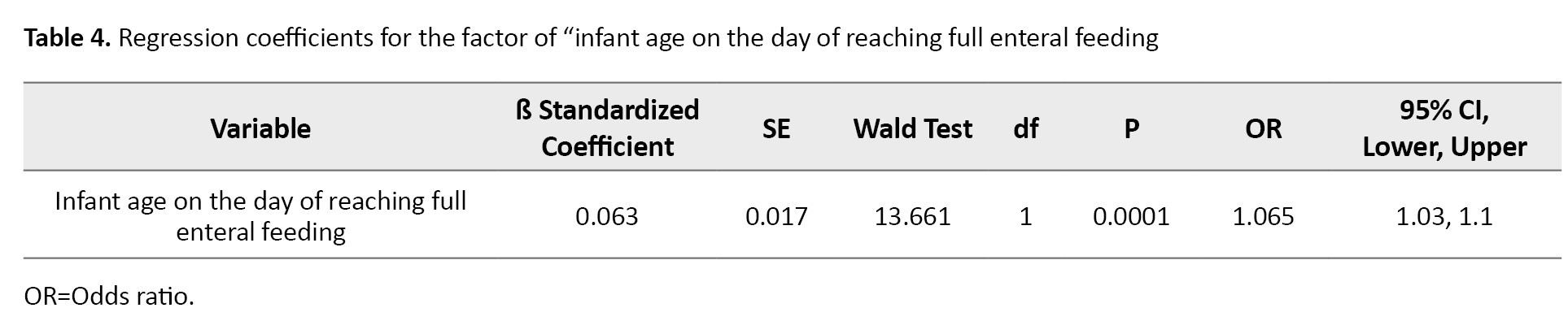
The variables that were significantly different between infants with positive and negative blood cultures were entered into the logistic regression model. Logistic regression with the likelihood ratio test was performed on all the covariates that had a significant effect. Only the variable of “infant age on the day of reaching full enteral feeding” remained in the model and was found to be significant. For every one day increase in the infant age on the day of reaching full enteral feeding, the risk of having a positive blood culture test for CLABSI increases by 0.06 units (Table 4).
Discussion
Our research results showed that the incidence rate of CRBSIs was 7.6 in 1000 catheters/days. This finding is consistent with those of Dubbink-Verheij et al., who reported a CRBSI incidence rate of 5.3 in 1000 catheters/days in infants with central venous catheters [15]. In another study, the incidence rate of CRBSIs ranged from 4.4 to 6.4 in 1000 catheters/days in infants weighing less than 1000 g [16]. Hu et al. reported a CRBSI rate of 10.6 in 1000 catheters/days in 381 infants with PICCs [7]. Jansen et al., in a study conducted from 2012 to 2020, reported a CRBSI rate of 8.8-25.23 in 1000 catheters/days in preterm infants [17]. Njere et al. showed a CRBSI rate of 17 in 1,000 catheter/days [18], which is higher than the rate reported in our study. Khieosanuk et al. reported a CRBSI rate of 3.32 in 1000 catheters/days [19], which is lower than the CRBSI rate in our study. The different results reported in other studies clearly show the impact of care on the incidence of CRBSIs and, thus, the need to plan for better care to reduce infections. The statistics should be collected periodically because the proper planning for reducing CRBSIs relies on accurate data.
Different CLABSI-associated factors were investigated in the study. One was the total length of hospital stay, which was significantly associated with PICC-related CLABSI in the present study. Tavanaee Sani et al. demonstrated that CLBSI increased the length of hospital stay from 4.2 to 5.7 days and raised the mortality rate [20]. In Stoll et al.’s study, the infants with late-onset sepsis had a longer length of hospital stay compared to infants without sepsis [21]. Nielsen’s study also indicated that a longer NICU stay is associated with a higher incidence of CRBSIs. Their results suggest that the occurrence of CRBSIs contributes to the prolonged hospitalization of infants with PICCs [22]. Further studies focusing on this aspect may provide more insight into the exact relationship between these variables.
Another factor associated with PICC-related CLABSI in the present study was the catheter dwell time. Milstone et al.’s study demonstrated that a dwell time of more than 7 days was associated with an increased risk of CRBSIs [23]. This finding was also reported in other studies [7, 18, 21, 23]. Various studies have reported that in NICUs, PICC dwell time is an independent risk factor for CLABSI [24-26]. However, there are contradictory findings in a study that reported that catheter dwell time did not increase the incidence of CLABSI [27], possibly due to adequate nutritional support, reduced invasive procedures, and increased skin maturity in children. Some studies have indicated that regular catheter replacement does not reduce the risk of CLABSI [28, 29]. Different CLABSI rates during different periods following PICC insertion highlight the complexity of this relationship [30]. Therefore, the studies suggest that catheter dwell time is likely an important variable affecting the CRBSI rate, but other factors can influence it and may increase or decrease accordingly. Further research is needed to explore the complexities of this relationship.
The variable of antibiotic therapy duration was also associated with PICC-related CLABSI in the present study. Jansen et al. found that, although there was no change in the reported rate of CLABSI related to PICCs over time, the use of antibiotics decreased [17]. The findings of Reynolds et al. [31] are against the results of the present study. Similarly, a study reported a 9.3% reduction in sepsis rates based on clinical symptoms in infants who received prophylactic antibiotics [32]. Bayoumi et al. found that impregnating the catheter with the antimicrobials before insertion had no significant effect on the reduction of CLABSI rate [33]. These results suggest that, not only the PICC-related CLABSI rate is likely associated with antibiotic therapy, but also prophylactic antibiotic therapy may yield better results. In the present study, antibiotic therapy was recorded and assessed over time. Interventional studies may be needed to obtain more accurate results in this regard.
The duration of mechanical ventilation at the catheter dwell time was another factor significantly associated with PICC-related CLABSI in the present study. A study found that effective infection control interventions reduced PICC-related CLABSI and ventilator-associated pneumonia [34]. However, it is important to note that infants dependent on mechanical ventilation are likely to be in poor conditions and may stay in the hospital for several days, which can contribute to a longer PICC dwell time. Since both the length of hospitalization and the catheter dwell time were identified as significant factors related to CLABSI, they may have a synergistic effect on each other.
Our result showed that only the infant age on the day of reaching full enteral feeding was the significant predictor of PICC-related CLABSI in infants. In a study that examined the predictors of PICC-related CLABSI, the nurse-to-patient ratio in specialized care units was identified as the only predictive factor [35]. In Badheka et al.’s study, two factors of longer external catheter length outside the body and the catheter placement in the operating room were reported as predictive factors for the increased incidence of PICC-related CLABSI in children [36]. It seems necessary to conduct further studies on the predictors of PICC-related CLABSI in infants.
The retrospective design and the distortion and incompleteness of some items in the infants’ files, such as the number of catheter dressings, were identified as limitations of the research. Overall, it can be concluded that the PICC-related CLABSI rate in infants can be influenced by multiple factors such as the duration of mechanical ventilation at the catheter dwell time, duration of antibiotic therapy, catheter dwell time, and total length of hospital stay. The infant age on the day of reaching full enteral feeding is the predictor of PICC-related CLABSI in infants. The findings can help in understanding the causes and factors related to PICC-related CLABSI in infants. More studies in Iran are recommended on the role of other variables, and longitudinal and systematic review studies should be conducted in this field.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.PHARMACY.REC.1402.148).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Authors' contributions
Conceptualization and supervision: Hamide Jalali, Maryam Varzeshnejad, and Elahe Rastkar Mehrabani; Methodology: Azam Shirinabadi Farahani; Data collection: Hamide Jalali, and Zahra Roosta; Data analysis: Maliheh Nasiri; Literature review: Hamide Jalali, Elahe Rastkar Mehrabani; Writing the original draft: Maryam Varzeshnejad and Hamide Jalali; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Mahdiyeh Teaching Hospital for Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
The neonatal period, the first 28 days of life, is the most vulnerable time for an individual’s survival [1]. Although the mortality rate in the neonatal period is nearly 30 times higher than in later stages of life [2], advancements in neonatology science have led to unprecedented improvements in neonatal survival [3]. With the increase in the survival of new-borns, the importance of vascular access in new-borns has also increased [4]. The use of peripheral vascular catheters is a common and invasive procedure in new-borns and is one of the most frequently performed painful procedures in neonatal care [5]. Routine vein access methods can have complications [6]. Repeated attempts to access peripheral veins can compromise the delicate skin of new-borns (which serves as the first line of defence against systemic infections) and puts the infant at risk of further injury [7].
The placement of a peripherally inserted central catheter (PICC) is a safe, convenient, and effective vascular access method due to its simplicity, high success rate, low complications, and the ability to administer hyperosmolar substances. It can reduce the excessive painful stimuli caused by repeated venepunctures in babies, ensuring timely intravenous nutrition and medication administration. However, the use of PICCs is associated with the risk of central line-associated bloodstream infection (CLABSI). These complications may even more occur in new-borns with severe illnesses [8]. The rate of CLABSI in new-borns is in a range of 0.3-8.21 per 1000 catheter/days. The CLABSI is among the most significant causes of late-onset sepsis [9]. Therefore, preventing CLABSIs resulted from central venous catheterization is a top priority in neonatal care units, as it can reduce mortality, morbidity, and the length of hospital stays.
Several studies have attempted to identify the causes and factors associated with CLABSI in new-borns with PICCs, but these factors have not been definitively established yet [4, 10, 11]. Various variables such as weight, gender, gestational age [11], catheter dwell time, site of insertion, and the number of tries for catheter insertion have been introduced as potential factors associated with catheter-related infections [4]. The type of catheter, catheter tip position, gestational age, and birth weight are other risk factors, although there is no clear consensus on which factor truly affects the occurrence of this infection [12]. Trained nurses are responsible for PICC placement in clinical settings [13]. In the nursing interventions classification (NIC) system, PICC placement and care are considered a standardized nursing intervention [14]. This research aims to investigate the rate of CLABSI related to PICC in the neonatal intensive care units (NICUs) of hospitals in Tehran, Iran, and identify the associated factors.
Materials and Methods
This is a retrospective analytical study with a cross-sectional design. The medical records of all infants with PICC admitted to two NICUs of one maternal and neonatal hospital in Tehran, Iran, over 5 years (2018-2023) were assessed, and the data of 321 cases that met the inclusion criteria were extracted. The inclusion criteria were having a PICC during hospitalization for more than 24 hours. The infants with catheters transferred to other hospitals for continued treatment (catheter removal in another hospital) were excluded.
For data collection, a demographic/clinical checklist was designed after identifying all possible factors associated with PICC-related infection from previous studies. The researcher-made checklist included three sections: a) Demographic/clinical information related to the infant (sex, birth weight, gestational age, cause of hospitalization, weight on the day of PICC insertion, post-natal age on the day of catheter insertion, weight on the day of PICC removal, age on the day of full enteral feeding, total days of hospitalization, days of mechanical ventilation at the catheter dwell time, days of antibiotic therapy days at the catheter dwell time); b) Information related to the catheter (catheter size, neonatal age at the catheterization time, number of catheterization attempts, site of insertion, location of catheter tip after insertion, dwell time, cause of catheter removal, and type of catheter-related complications), and c) Information related to CLABSI, including laboratory test results. The validity of the checklist was confirmed based on a content validity ratio of 0.87 and a content validity index of 0.88, according to the opinions of 10 nursing faculty members. The Mann-Whitney U test and the logistic regression analysis were used for data analysis in SPSS software, version 20.
Results
Of 321 infants with PICC, 168(52.3%) were male and 153(47.6%) were female. Their mean birth weight was 1219.23±447.68 g, while their mean weight on the day of PICC insertion was 1215.72±454.81 g, and the mean weight on the day of PICC removal was 1348.95±481.46 g, which was not significantly different from the weight on the day of PICC insertion. These results indicate that the insertion of PICC was mostly done for infants with weight <1500 g, and the infants had weight <1500 g until the time of PICC removal. The mean gestational age of the infants was 29.2±2.7 weeks, indicating that most of the infants were preterm. The mean post-natal age of the infants on the day of catheter insertion was 6.79±11.38. It also showed that most infants had catheter insertion in the first week of life.
The mean catheter dwell time was 15.97±10.45 days, and the total days of hospitalization was 40.9±24.7. The most common clinical diagnosis for infants was sepsis (49.53%). None of the infants had the clinical diagnosis of CLABSI. For 84.1% of the infants, a size 1 catheter had been inserted. Moreover, 62.3% of the infants had their PICC inserted at the first attempt. In 77.8% of cases, the catheter was placed in the upper extremities. Additionally, 85% of infants were under mechanical ventilation. The most common cause of catheter removal was “complications” (about 40%). In most cases, the catheter insertion was performed by a PICC team member (88.7%). Almost all infants (98.7%) were under antibiotic therapy at the time of catheter insertion. The majority of infants (25.86%) had an Apgar score of 9 at 1 minute, and 37% of infants had an Apgar score of 10 at 5 minutes. Other information is presented in Tables 1 and 2.



The rate of CLABSI caused by PICC was 7.60 per 1000 catheters/days. The Mann-Whitney U test results showed statistically significant differences between infants with positive blood culture test for CLABS and those with negative blood cultures in terms of total days of hospitalization (P=0.007), antibiotics therapy at the catheter dwell time (P=0.005), infant age on the day of reaching full enteral feeding (P=0.002), mechanical ventilation at the catheter dwell time (P=0.007), cause of catheter removal (P=0.001), previous history of PICC insertion (P=0.003), catheter dwell time (P=0.03) and duration of antibiotic therapy (P=0.005). In the rest of the variables, no significant differences were found between infants with positive and negative blood cultures (Table 3).

The variables that were significantly different between infants with positive and negative blood cultures were entered into the logistic regression model. Logistic regression with the likelihood ratio test was performed on all the covariates that had a significant effect. Only the variable of “infant age on the day of reaching full enteral feeding” remained in the model and was found to be significant. For every one day increase in the infant age on the day of reaching full enteral feeding, the risk of having a positive blood culture test for CLABSI increases by 0.06 units (Table 4).

Discussion
Our research results showed that the incidence rate of CRBSIs was 7.6 in 1000 catheters/days. This finding is consistent with those of Dubbink-Verheij et al., who reported a CRBSI incidence rate of 5.3 in 1000 catheters/days in infants with central venous catheters [15]. In another study, the incidence rate of CRBSIs ranged from 4.4 to 6.4 in 1000 catheters/days in infants weighing less than 1000 g [16]. Hu et al. reported a CRBSI rate of 10.6 in 1000 catheters/days in 381 infants with PICCs [7]. Jansen et al., in a study conducted from 2012 to 2020, reported a CRBSI rate of 8.8-25.23 in 1000 catheters/days in preterm infants [17]. Njere et al. showed a CRBSI rate of 17 in 1,000 catheter/days [18], which is higher than the rate reported in our study. Khieosanuk et al. reported a CRBSI rate of 3.32 in 1000 catheters/days [19], which is lower than the CRBSI rate in our study. The different results reported in other studies clearly show the impact of care on the incidence of CRBSIs and, thus, the need to plan for better care to reduce infections. The statistics should be collected periodically because the proper planning for reducing CRBSIs relies on accurate data.
Different CLABSI-associated factors were investigated in the study. One was the total length of hospital stay, which was significantly associated with PICC-related CLABSI in the present study. Tavanaee Sani et al. demonstrated that CLBSI increased the length of hospital stay from 4.2 to 5.7 days and raised the mortality rate [20]. In Stoll et al.’s study, the infants with late-onset sepsis had a longer length of hospital stay compared to infants without sepsis [21]. Nielsen’s study also indicated that a longer NICU stay is associated with a higher incidence of CRBSIs. Their results suggest that the occurrence of CRBSIs contributes to the prolonged hospitalization of infants with PICCs [22]. Further studies focusing on this aspect may provide more insight into the exact relationship between these variables.
Another factor associated with PICC-related CLABSI in the present study was the catheter dwell time. Milstone et al.’s study demonstrated that a dwell time of more than 7 days was associated with an increased risk of CRBSIs [23]. This finding was also reported in other studies [7, 18, 21, 23]. Various studies have reported that in NICUs, PICC dwell time is an independent risk factor for CLABSI [24-26]. However, there are contradictory findings in a study that reported that catheter dwell time did not increase the incidence of CLABSI [27], possibly due to adequate nutritional support, reduced invasive procedures, and increased skin maturity in children. Some studies have indicated that regular catheter replacement does not reduce the risk of CLABSI [28, 29]. Different CLABSI rates during different periods following PICC insertion highlight the complexity of this relationship [30]. Therefore, the studies suggest that catheter dwell time is likely an important variable affecting the CRBSI rate, but other factors can influence it and may increase or decrease accordingly. Further research is needed to explore the complexities of this relationship.
The variable of antibiotic therapy duration was also associated with PICC-related CLABSI in the present study. Jansen et al. found that, although there was no change in the reported rate of CLABSI related to PICCs over time, the use of antibiotics decreased [17]. The findings of Reynolds et al. [31] are against the results of the present study. Similarly, a study reported a 9.3% reduction in sepsis rates based on clinical symptoms in infants who received prophylactic antibiotics [32]. Bayoumi et al. found that impregnating the catheter with the antimicrobials before insertion had no significant effect on the reduction of CLABSI rate [33]. These results suggest that, not only the PICC-related CLABSI rate is likely associated with antibiotic therapy, but also prophylactic antibiotic therapy may yield better results. In the present study, antibiotic therapy was recorded and assessed over time. Interventional studies may be needed to obtain more accurate results in this regard.
The duration of mechanical ventilation at the catheter dwell time was another factor significantly associated with PICC-related CLABSI in the present study. A study found that effective infection control interventions reduced PICC-related CLABSI and ventilator-associated pneumonia [34]. However, it is important to note that infants dependent on mechanical ventilation are likely to be in poor conditions and may stay in the hospital for several days, which can contribute to a longer PICC dwell time. Since both the length of hospitalization and the catheter dwell time were identified as significant factors related to CLABSI, they may have a synergistic effect on each other.
Our result showed that only the infant age on the day of reaching full enteral feeding was the significant predictor of PICC-related CLABSI in infants. In a study that examined the predictors of PICC-related CLABSI, the nurse-to-patient ratio in specialized care units was identified as the only predictive factor [35]. In Badheka et al.’s study, two factors of longer external catheter length outside the body and the catheter placement in the operating room were reported as predictive factors for the increased incidence of PICC-related CLABSI in children [36]. It seems necessary to conduct further studies on the predictors of PICC-related CLABSI in infants.
The retrospective design and the distortion and incompleteness of some items in the infants’ files, such as the number of catheter dressings, were identified as limitations of the research. Overall, it can be concluded that the PICC-related CLABSI rate in infants can be influenced by multiple factors such as the duration of mechanical ventilation at the catheter dwell time, duration of antibiotic therapy, catheter dwell time, and total length of hospital stay. The infant age on the day of reaching full enteral feeding is the predictor of PICC-related CLABSI in infants. The findings can help in understanding the causes and factors related to PICC-related CLABSI in infants. More studies in Iran are recommended on the role of other variables, and longitudinal and systematic review studies should be conducted in this field.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.PHARMACY.REC.1402.148).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Authors' contributions
Conceptualization and supervision: Hamide Jalali, Maryam Varzeshnejad, and Elahe Rastkar Mehrabani; Methodology: Azam Shirinabadi Farahani; Data collection: Hamide Jalali, and Zahra Roosta; Data analysis: Maliheh Nasiri; Literature review: Hamide Jalali, Elahe Rastkar Mehrabani; Writing the original draft: Maryam Varzeshnejad and Hamide Jalali; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Mahdiyeh Teaching Hospital for Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- Boztepe H, Inci F, Tanhan F. Posttraumatic growth in mothers after infant admission to neonatal intensive care unit. Paediatr Croat. 2015; 59(1):14-8. [DOI:10.13112/PC.2015.3]
- Al-Shahethi AH, Bulgiba A, Zaki RA, Al-Dubai SAR, Al-Surimi KM, Al-Serouri AA. Neonatal mortality in the eastern Mediterranean region: Socio-demographic, economic and perinatal factors, 1990-2013. Iran J Pediatr. 2018; 28(1):e10485. [DOI:10.5812/ijp.10485]
- Parikh P, Juul SE. Neuroprotection strategies in preterm encephalopathy. Semin Pediatr Neurol. 2019; 32:100772. [DOI:10.1016/j.spen.2019.08.008] [PMID]
- Ferreira J, Camargos PAM, Rosado V, Mourão PHO, Romanelli RMC. Risk factors for central venous catheter-related bloodstream infection in neonates. Am J Infect Control. 2020; 48(9):1102-3. [DOI:10.1016/j.ajic.2019.12.004] [PMID]
- Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006; 117(3 Pt 2):S9-S22. [DOI:10.1542/peds.2005-0620C] [PMID]
- Fleiss N, Klein-Cloud R, Gill B, Feldstein NA, Fallon EM, Ruzal-Shapiro C, et al. Subdural extravasation of crystalloids and blood products through a scalp peripheral intravenous catheter into the subdural space of a neonate on veno-arterial extracorporeal membrane oxygenation. J Neonatal Perinatal Med. 2021;14(4):601-5. [DOI:10.3233/npm-200610] [PMID]
- Hu Y, Ling Y, Ye Y, Zhang L, Xia X, Jiang Q, et al. Analysis of risk factors of PICC-related bloodstream infection in newborns: Implications for nursing care. Eur J Med Res. 2021; 26(1):80. [DOI:10.1186/s40001-021-00546-2] [PMID]
- Li R, Cao X, Shi T, Xiong L. Application of peripherally inserted central catheters in critically ill newborns experience from a neonatal intensive care unit. Medicine. 2019; 98(32):e15837. [DOI:10.1097/MD.0000000000015837] [PMID]
- Hightower HB, Young JA, Thomas J, Smith JJ, Hobby-Noland D, Palombo G, et al. Reduction of Central-line-Associated Bloodstream Infections in a Tertiary Neonatal Intensive Care Unit through simulation education. Pediatr Qual Saf. 2022; 7(6):e610. [DOI:10.1097/pq9.0000000000000610] [PMID]
- Cheng HY, Lu CY, Huang LM, Lee PI, Chen JM, Chang LY. Increased frequency of peripheral venipunctures raises the risk of central-line associated bloodstream infection in neonates with peripherally inserted central venous catheters. J Microbiol Immunol Infect. 2016; 49(2):230-6. [DOI:10.1016/j.jmii.2014.06.001] [PMID]
- Hsu JF, Tsai MH, Huang HR, Lien R, Chu SM, Huang CB. Risk factors of catheter-related bloodstream infection with percutaneously inserted central venous catheters in very low birth weight infants: A center’s experience in Taiwan. Pediatr Neonatol. 2010; 51(6):336-42. [DOI:10.1016/S1875-9572(10)60065-4] [PMID]
- Wen J, Yu Q, Chen H, Chen N, Huang S, Cai W. Peripherally inserted central venous catheter-associated complications exert negative effects on body weight gain in neonatal intensive care units. Asia Pac J Clin Nutr. 2017; 26(1):1-5. [PMID]
- Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infusion Nurs. 2008; 31(3):165-76. [DOI:10.1097/01.NAN.0000317703.66395.b8] [PMID]
- Wagner CM, Butcher HK, Bulechek GM, Dochterman JM, Clarke MF. Nursing Interventions Classification (NIC)-E-Book. Amsterdam: Elsevier Health Sciences; 2023. [Link]
- Dubbink-Verheij GH, Bekker V, Pelsma IC, van Zwet EW, Smits-Wintjens VE, Steggerda SJ, et al. Bloodstream infection incidence of different central venous catheters in neonates: A descriptive cohort study. Front Pediatr. 2017; 5:142. [DOI:10.3389/fped.2017.00142]
- Edwards JR, Peterson KD, Andrus ML, Tolson JS, Goulding JS, Dudeck MA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2006, issued June 2007. Am J Infect Control. 2007; 35(5):290-301. [DOI:10.1016/j.ajic.2007.04.001] [PMID]
- Jansen SJ, van der Hoeven A, van den Akker T, Veenhof M, von Asmuth EG, Veldkamp KE, et al. A longitudinal analysis of nosocomial bloodstream infections among preterm neonates. Eur J Clin Microbiol Infect Dis. 2022; 41(11):1327-36. [DOI:10.1007/s10096-]
- Njere I, Islam S, Parish D, Kuna J, Keshtgar AS. Outcome of peripherally inserted central venous catheters in surgical and medical neonates. J Pediatr Surg. 2011; 46(5):946-50. [DOI:10.1016/j.jpedsurg.2011.02.037] [PMID]
- Khieosanuk K, Fupinwong S, Tosilakul A, Sricharoen N, Sudjaritruk T. Incidence rate and risk factors of central line-associated bloodstream infections among neonates and children admitted to a tertiary care university hospital. Am J Infect Control. 2022; 50(1):105-7. [DOI:10.1016/j.ajic.2021.07.016] [PMID]
- Tavanaee Sani A, Eslami Nowkandeh AR, Ghorbany H. [Central venous catheter related infection among patients on hemodialysis (Persian)]. Med J Mashhad Univ Medical Sci. 2012; 55(2):110-5. [DOI: 10.22038/mjms.2012.5298]
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002; 110(2):285-91. [DOI:10.1542/peds.110.2.285] [PMID]
- Nielsen CL, Zachariassen G, Holm KG. Central line-associated bloodstream infection in infants admitted to a level lllneonatal intensive care unit. Dan Med J. 2022; 69(5):A05210463. [PMID]
- Milstone AM, Reich NG, Advani S, Yuan G, Bryant K, Coffin SE, et al. Catheter dwell time and CLABSIs in neonates with PICCs: A multicenter cohort study. Pediatrics. 2013; 132(6):e1609-e15. [DOI:10.1542/peds.2013-1645] [PMID]
- Rainey SC, Deshpande G, Boehm H, Camp K, Fehr A, Horack K, et al. Development of a pediatric PICC team under an existing sedation service: A 5-year experience. Clin Med Insights Pediatr. 2019; 13:1179556519884040. [DOI:10.1177/1179556519884040] [PMID]
- Li X, Ding X, Shi P, Zhu Y, Huang Y, Li Q, et al. Clinical features and antimicrobial susceptibility profiles of culture-proven neonatal sepsis in a tertiary children’s hospital, 2013 to 2017. Medicine. 2019; 98(12):e14686. [DOI:10.1097/MD.0000000000014686] [PMID]
- Campagna S, Gonella S, Berchialla P, Morano G, Rigo C, Zerla PA, et al. Can peripherally inserted central catheters be safely placed in patients with cancer receiving chemotherapy? A retrospective study of almost 400,000 catheter-days. Oncol. 2019; 24(9):e953-e9. [DOI:10.1634/theoncologist.2018-0281] [PMID]
- LaRusso K, Schaack G, Fung T, McGregor K, Long J, Dumas MP, et al. Should you pick the PICC? Prolonged use of peripherally inserted central venous catheters in children with intestinal failure. J Pediatr Surg. 2019; 54(5):999-1004. [DOI:10.1016/j.jpedsurg.2019.01.052] [PMID]
- Hon K, Bihari S, Holt A, Bersten A, Kulkarni H. Rate of catheter-related bloodstream infections between tunneled central venous catheters versus peripherally inserted central catheters in adult home parenteral nutrition: A meta-analysis. JPEN J Parenter Enteral Nutr. 2019; 43(1):41-53. [DOI:10.1002/jpen.1421] [PMID]
- Berger R, Messina AF, Chandler NM, Amankwah EK, Shaw PH. Instituting a new central line policy to decrease central line-associated blood stream infection rates during induction therapy in pediatric acute lymphoblastic leukemia patients. J Pediatr Hematol Oncol. 2020; 42(7):433-7. [DOI:10.1097/MPH.0000000000001748] [PMID]
- Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics. 2010; 125(4):648-53. [DOI:10.1542/peds.2009-2559] [PMID]
- Reynolds GE, Tierney SB, Klein JM. Antibiotics before removal of percutaneously inserted central venous catheters reduces clinical sepsis in premature infants. J Pediatr Pharmacol Ther. 2015; 20(3):203-9. [DOI:10.5863/1551-6776-20.3.203] [PMID]
- Yan PR, Chi H, Chiu NC, Huang CY, Huang DT, Chang L, et al. Reducing catheter related bloodstream infection risk of infant with a prophylactic antibiotic therapy before removing peripherally inserted central catheter: A retrospective study. J Microbiol Immunol Infect. 2022; 55(6 Pt 2):1318-25. [DOI:10.1016/j.jmii.2021.09.016] [PMID]
- Bayoumi MAA, van Rens MFPT, Chandra P, Masry A, D'Souza S, Khalil AM, et al. Does the antimicrobial-impregnated peripherally inserted central catheter decrease the CLABSI rate in neonates? Results from a retrospective cohort study. Front Pediatr. 2022; 10:1012800. [DOI:10.3389/fped.2022.1012800] [PMID]
- Smulders CA, van Gestel JP, Bos AP. Are central line bundles and ventilator bundles effective in critically ill neonates and children? Intensive Care Med. 2013; 39(8):1352-8. [DOI:10.1007/s00134-013-2927-7] [PMID]
- Aloush SM, Alsaraireh FA. Nurses’ compliance with central line associated blood stream infection prevention guidelines. Saudi Med J. 2018; 39(3):273-9. [DOI:10.15537/smj.2018.3.21497] [PMID]
- Badheka A, Bloxham J, Schmitz A, Freyenberger B, Wang T, Rampa S, et al. Outcomes associated with peripherally inserted central catheters in hospitalised children: A retrospective 7-year single-centre experience. BMJ open. 2019; 9(8):e026031. [DOI:10.1136/bmjopen-2018-026031] [PMID]
Article Type : Research |
Subject:
General
Received: 2024/01/6 | Accepted: 2024/10/15 | Published: 2025/01/12
Received: 2024/01/6 | Accepted: 2024/10/15 | Published: 2025/01/12
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



