Mon, Dec 29, 2025
Volume 35, Issue 3 (6-2025)
JHNM 2025, 35(3): 228-238 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Malekzadeh J, Ghouchani M, Sadeghi T, Bagherimoghadam A, Rajabpour M. The Effects of the ABCDE Bundle on the Hemodynamic Stability of Patients under Mechanical Ventilation. JHNM 2025; 35 (3) :228-238
URL: http://hnmj.gums.ac.ir/article-1-2254-en.html
URL: http://hnmj.gums.ac.ir/article-1-2254-en.html
Javad Malekzadeh1 

 , Mahsa Ghouchani2
, Mahsa Ghouchani2 

 , Tahereh Sadeghi3
, Tahereh Sadeghi3 

 , Ahmad Bagherimoghadam4
, Ahmad Bagherimoghadam4 

 , Mohammad Rajabpour *5
, Mohammad Rajabpour *5 




 , Mahsa Ghouchani2
, Mahsa Ghouchani2 

 , Tahereh Sadeghi3
, Tahereh Sadeghi3 

 , Ahmad Bagherimoghadam4
, Ahmad Bagherimoghadam4 

 , Mohammad Rajabpour *5
, Mohammad Rajabpour *5 


1- Instructor, Department of Medical Emergencies, Nursing and Midwifery Care Research Center, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Nursing (MSc.), Department of Critical Care Nursing, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Associate Professor, Department of Pediatrics, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Associate Professor, Department of Anesthesiology, School of Medicine, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
5- PhD. Candidate of Nursing, Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran. ,Rajabpoorm871@gmail.com
2- Nursing (MSc.), Department of Critical Care Nursing, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Associate Professor, Department of Pediatrics, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Associate Professor, Department of Anesthesiology, School of Medicine, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad, Iran.
5- PhD. Candidate of Nursing, Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Keywords: Mechanical ventilation, Intensive Care Units (ICUs), Breathing exercises, Delirium, Exercise
Full-Text [PDF 586 kb]
(213 Downloads)
| Abstract (HTML) (530 Views)
Full-Text: (227 Views)
Introduction
Adult patients requiring mechanical ventilation in Intensive Care Units (ICUs) are increasing [1]. Mechanical ventilation improves ventilation, reduces breathing work, strengthens spontaneous breathing, and increases active breathing capacity [2]. Patients under mechanical ventilation undergo sedation to tolerate the endotracheal tube and long-term lying position, prevent fighting with the ventilator, tolerate many procedures, and optimize oxygen consumption [1]. Sedation and analgesia can help increase patient comfort, but it has a non-selective inhibition of blood circulation and breathing centers [3]. Mechanical ventilation may also cause hemodynamic instability in patients [4]. Common adverse cardiovascular responses to mechanical ventilation include barotrauma, lung injury, pneumonia, hemodynamic alterations and instability, myocardial ischemia, autonomic dysfunction, and cardiac dysrhythmias. Other problems include endotracheal tube complications, respiratory muscle weakness, and secretion retention [5].
Examining hemodynamic indicators is an important monitoring tool for critically ill patients. It can not only determine the real response of the body but also help in clinical interventions and appropriate treatment to improve tissue perfusion as soon as possible [6]. Therefore, there is an urgent need to implement interpersonal and evidence-based strategies to reduce the complications associated with long-term mechanical ventilation, long-term sedation, and improving hemodynamic status in adults under mechanical ventilation [7].
The ABCDE bundle (ABC: Awakening, Breathing, and Coordination, D: Delirium and Monitoring, E: Early Mobility) is a multidisciplinary, evidence-based approach that improves the prognosis of patients in the ICU by enhancing the quality of care and outcomes of patients under mechanical ventilation [8]. In the US, the implementation of the ABCDE bundle is supported not only by special care groups but also by national quality improvement organizations as a means to increase the quality and safety of critical care and improve the prognosis of patients [9]. Among the benefits of the ABCDE bundle are reducing the duration of mechanical ventilation, hospitalization, and the prevalence and duration of delirium [10]. Although the previous studies that implemented at least three of the ABCDE bundle components showed positive short-term benefits regarding the length of ICU stay and mortality [9, 10], they reported that bundle implementation did not necessarily reduce the incidence or duration of delirium [11].
The ABCDE bundle is safe and effective [11]. Still, its effects on central venous pressure, Heart Rate (HR), and oxygenation index (PaO2/FiO2) in patients undergoing mechanical ventilation have been reported in a descriptive cross-sectional manner [3]. Also, in previous studies [3, 10, 11], three components of the bundle have mainly been used, and there is limited evidence regarding the effectiveness of the full implementation of this bundle. Besides, its efficacy on the complications of mechanical ventilation has been given less attention, and the results of the studies are contradictory.
Therefore, this study was conducted as a clinical trial to determine the effect of using the ABCDE bundle on the stability of the hemodynamic indicators of patients under mechanical ventilation.
Materials and Methods
This clinical trial was conducted on 90 intubated patients under mechanical ventilation from July to December 2022. The sample size was calculated based on the study of Ren et al. [3]. For this purpose, 45 patients were estimated in each group, taking into account 90% power (1-β), 5% type 1 error (α), effect size of 10, standard deviation of 15, and the correlation between measurements of 0.8. Taking a 10% drop into account, about 100 patients were recruited for the study. The samples were divided into the intervention (n=46) and control groups (n=46) using a random block allocation method (25 block sizes of 4 on the sealed envelope website [12]). One patient in the intervention group and one in the control group were excluded due to not participating in the follow-up (Figure 1). Random concealment was done using the SNOSE (Sequentially Numbered, Opaque, Sealed Envelope) technique.
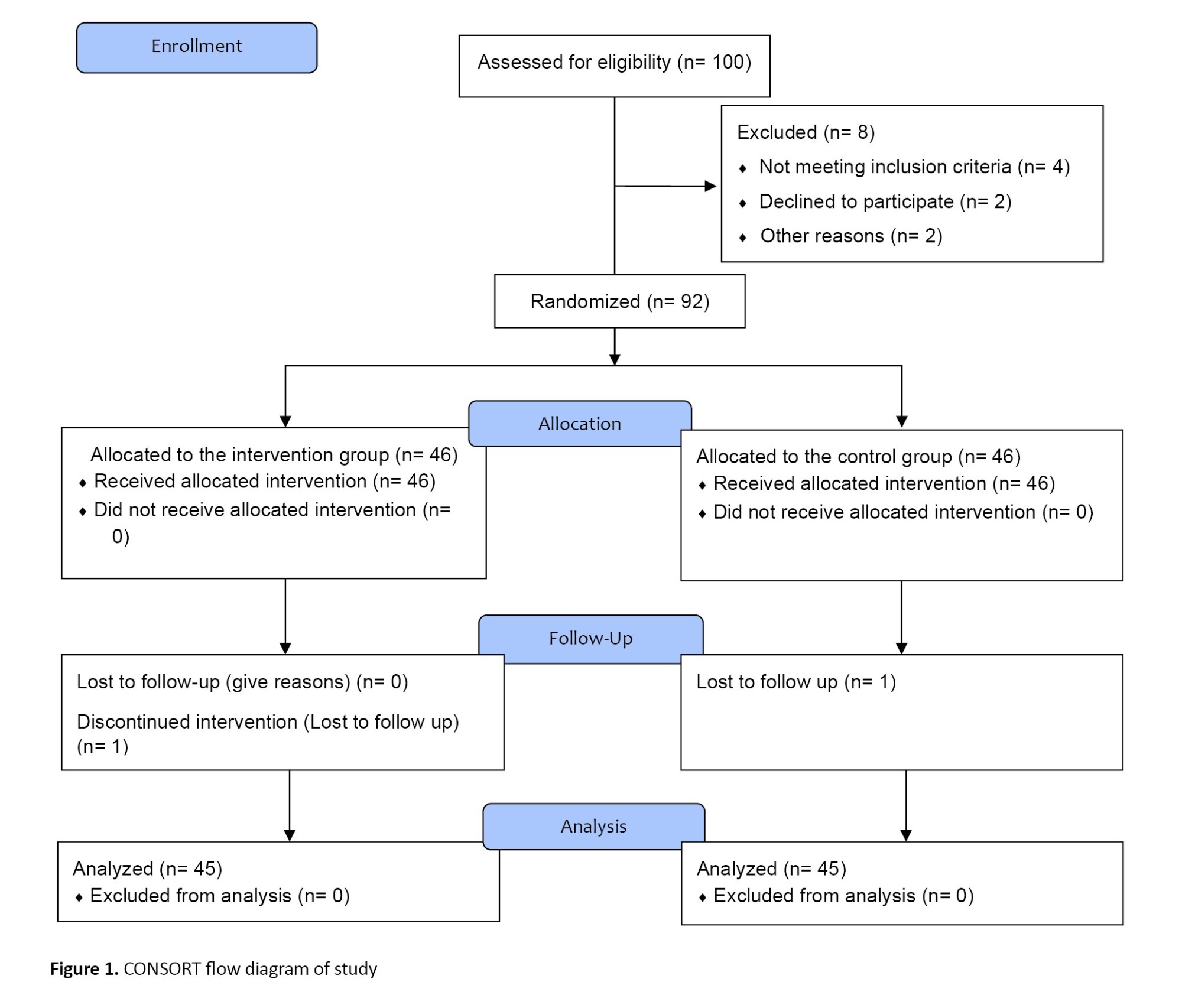
The inclusion criteria were as follows: Being age 18-60 years old, suffering from subgroups of pulmonary diseases (e.g. chronic obstructive pulmonary diseases, acute respiratory distress syndrome, pneumonia, and embolism) based on medical records, the minimum duration of mechanical ventilation and treatment time of sedation and analgesia ≥48 hours [13], the minimum length of stay in ICU ≥48 hours, no neuromuscular disease (such as Myasthenia Gravis (MG), Muscular-spinal Atrophy (SMA), based on medical record), no physical disorder, Richmond Agitation-sedation Scale (RASS) score ≥0. The exclusion criteria were as follows: Unwillingness to continue participation in research at any stage, patient’s death or transfer from the ICU, and lack of consciousness despite cessation of sedation.
The tools used in the present study were the demographic form, the RASS, the full outline of unresponsiveness (FOUR) score, and the researcher-made checklist of hemodynamic status. The demographic information form included questions about age, gender, marital status, past medical history, and smoking/drug abuse.
The patients’ hemodynamic indicators, including Mean Arterial Pressure (MAP), Pulse Pressure (PP), HR, and Blood Pressure (BP), were measured using Alborz B5 and B9 monitoring devices. The reliability of these devices was checked by regularly calibrating them. Additionally, the research team followed the manufacturer’s guidelines for properly using and maintaining the devices.
The RASS measures the level of restlessness in the ICU. This scale is a 10-point continuum from -5 to +4. The validity of this instrument has been confirmed by Ely and Sessler [14, 15]. The FOUR score scale is an international scale for monitoring the level of consciousness of intubated patients, and the validity and reliability of this tool have already been confirmed [16]. RASS and FOUR scores were measured in both groups of patients before the intervention.
The ABCDE bundle was used in the intervention group. The implementation of the ABCDE bundle was as follows. ABC includes awakening and breathing coordination, and has 4 stages [17]. In the first step, a screening for the Spontaneous Awakening Trial (SAT) safety is done. If the screening is deemed safe, step 2, which involves “doing SAT,” will be carried out, and the administration of sedatives will be discontinued under the supervision of the attending physician.
In the second stage, sedation is resumed with half the previous dose, and start the first stage is started again 24 hours later. If step 2 is successful, step 3 is ignited. The researcher performs the spontaneous breathing trial (SBT) safety screening in this stage according to the protocol. If the failure criteria appear in this stage, stage 1 is repeated 24 hours later.
If step 3 was successful, step 4 is initiated, and “SBT intervention” is performed. Implementing SBT involves discontinuing mechanical ventilation support and using a ventilator with a respiratory rate set at zero, positive end-expiratory pressure/continuous positive airway pressure ≤5, pressure support ventilation ≤5, and a T-tube. This intervention is deemed successful if the patient tolerates spontaneous breathing for 30-120 minutes [18] and the anesthesiologist prescribes extubating. In the event of failure in stage 4, full respiratory support is provided for the patient.
Every morning, the Delirium (D) in patients on mechanical ventilation was assessed using the ICU D assessment diagnostic table. The progression of D was monitored, and the daily assessment scores of patients on mechanical ventilation were recorded. On this basis, the anesthesiologist adjusts the dose of the sedatives and analgesics used. The physicians further identified those diagnosed as positive.
For E stage (early exercise/mobility) and according to the conditions of patients on mechanical ventilation, if they meet the minimum criteria for early mobility, an activity was adopted: Sitting on the edge of the bed, standing by the bed, or sitting in a chair, walking a short distance. During the mobility, the criteria for stopping early mobility were considered according to the protocol [11].
Finally, hemodynamic indicators, including MAP, PP, and HR, were measured at the end of the interventions and after the doctor’s visit on days 0, 1, 3, 5, and 7.
In the control group, according to the routine of the ward, common sedation and pain relief were performed. The patient’s level of consciousness and hemodynamic indicators were measured after the doctor’s visit on days 0, 1, 3, 5, and 7. This process was performed for each patient throughout the week, and depending on the number of active beds in the hospital, 5 to 10 patients were studied each week.
Data analysis was done using SPSS software, version 20, and descriptive statistics, including frequency, Mean±SD, were calculated. Next, quantitative data regarding normal distribution were checked using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The independent t-test was used to compare two groups regarding normal quantitative variables, and the Mann-Whitney U test was used for non-normal quantitative variables (age, FOUR score, and RASS). Qualitative variables (gender, marital status, past medical history, and smoking/drug abuse) were also compared between the two groups using the chi-square test. A repeated-measure ANOVA test was used to compare the intra-groups. In all tests, P<0.05 was considered statistically significant.
Results
Patients’ Mean±SD ages were 48.76±11.46 in the intervention and 47.58±11.56 years in the control group. The result of the Mann-Whitney U test showed no statistically significant difference between the two groups in this respect. Also, the Mean±SD of the FOUR score scales were 7.78±1.24 in the intervention group patients and 8.00±1.10 in the control group. The result of the Mann-Whitney test showed no statistically significant difference between the two groups in this respect. The Mean±SD scores of the RASS scale were -2.16±0.71 in the intervention and -2.00±0.77 in the control group. The result of the Mann-Whitney U test showed no statistically significant difference between the two groups in this regard. The other demographic and disease characteristics of the two groups are listed in Table 1.
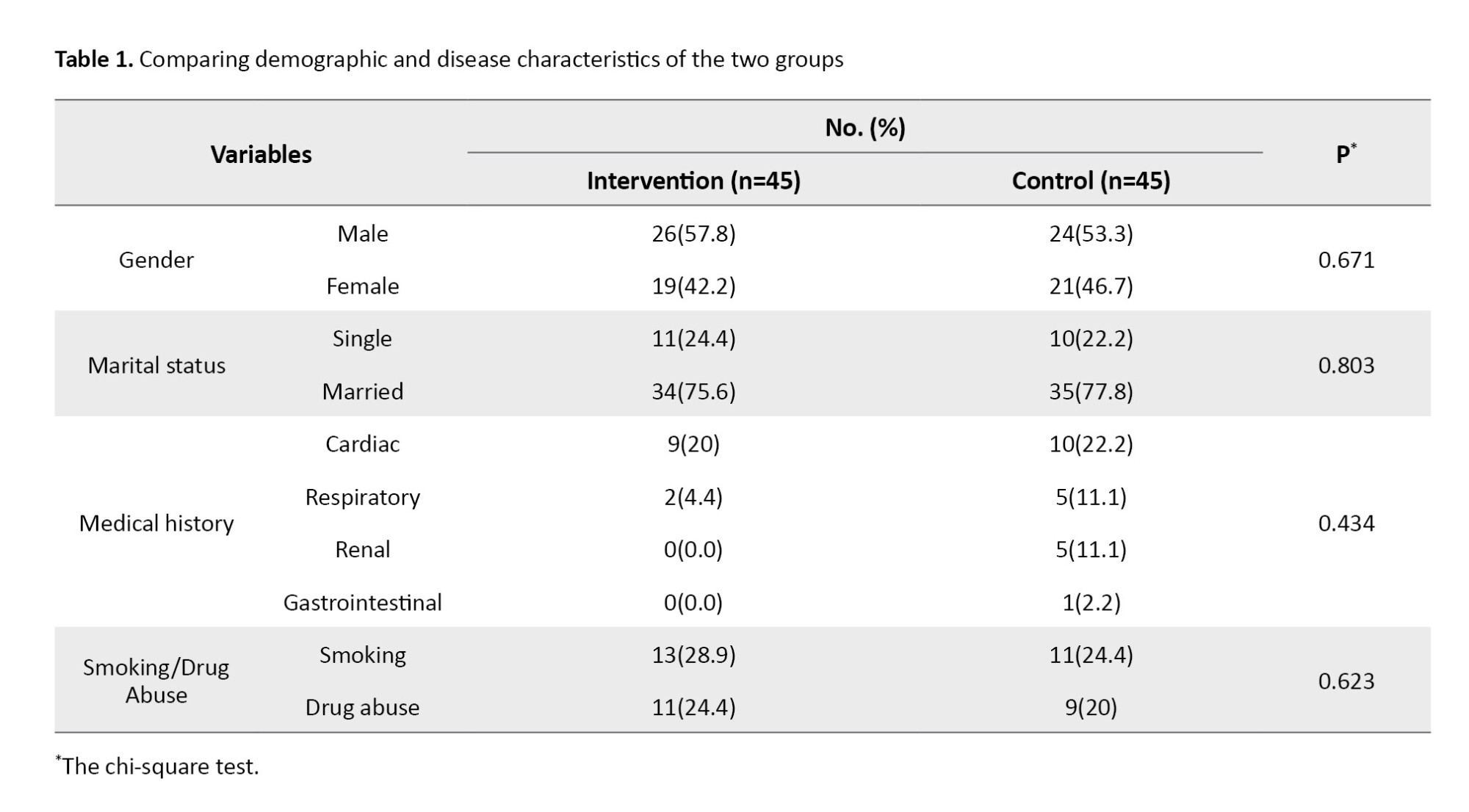
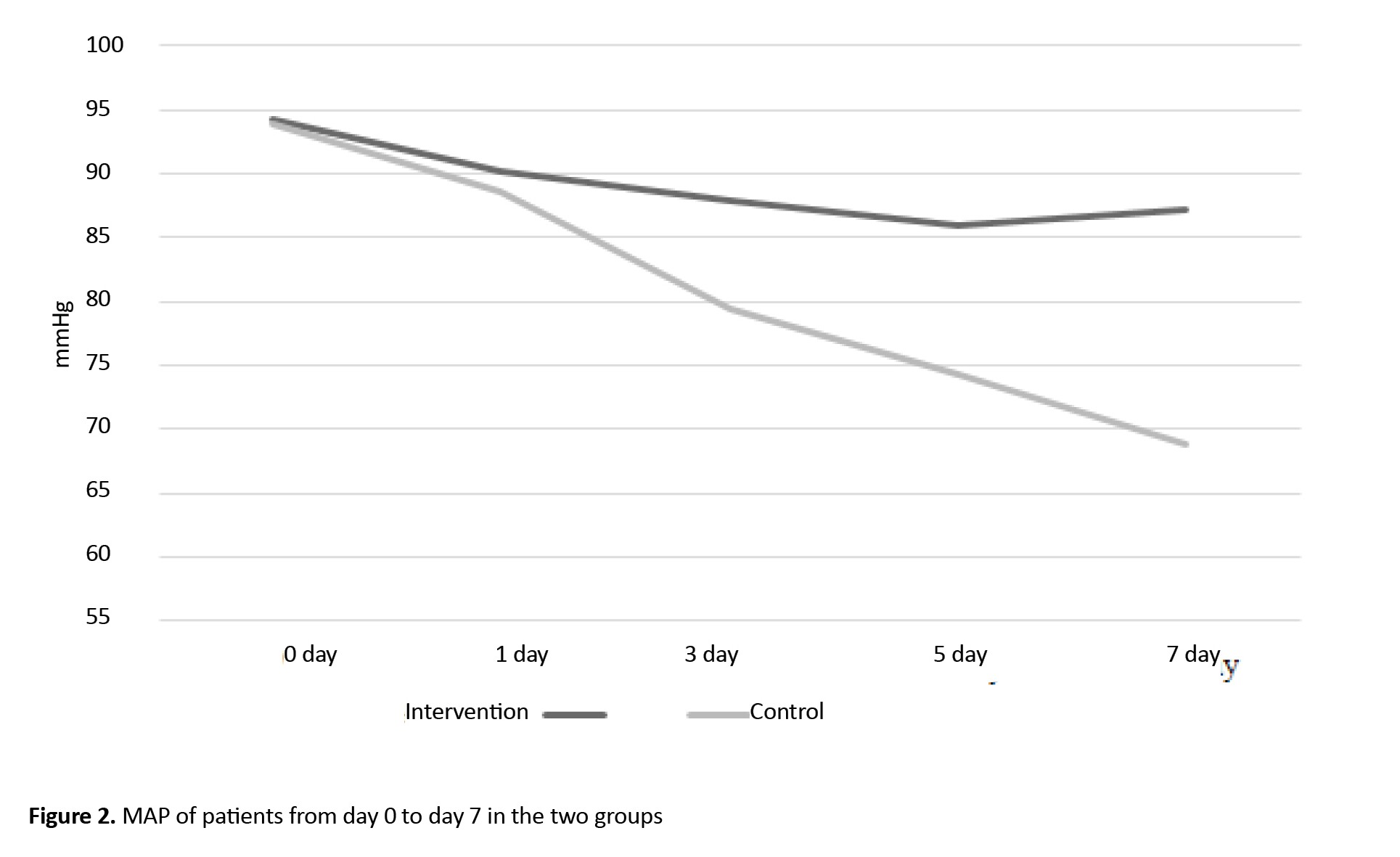
The result of the independent t-test showed that the mean MAP before the intervention (day 0) and the first day was not statistically significantly different in the two study groups. However, the Mean±SD MAP were 79.39±10.9 on the day 3, 74.2±13.6 on the day 5, and 68.7±14.8 on the day 7 in the control group, which showed a statistically significant decrease compared to the intervention group (P<0.05). Also, the repeated measures ANOVA results showed that the effects of time, group, and time×group on the mean MAP were statistically significant (P=0.0001). The post hoc (Bonferroni) test revealed that the mean MAP from day 3 to day 7 significantly decreased (P=0.0001). These results were shown in Table 2.
Figure 2 shows the changes in MAP over time in two groups.
The mean PP values showed no statistically significant difference before the intervention (day 0) and on days 1 to 3 in the patients of the two groups. However, the Mean±SD PP values were 56.04±11.11 on the day 5 and 58.92±6.81 on the day 7 in the intervention group. The independent t-test results showed that in the intervention group, from the 5 to the day 7, the mean PP value increased significantly compared to the control group (P=0.0001). The results of the repeated measures ANOVA demonstrated statistically significant effects of time, group, and time×group on the mean PP (P=0.012). Also, post hoc test (Bonferroni test) indicated a significant decrease in the mean PP value from day 5 to day 7 (P=0.001). These results were shown in Table 3.
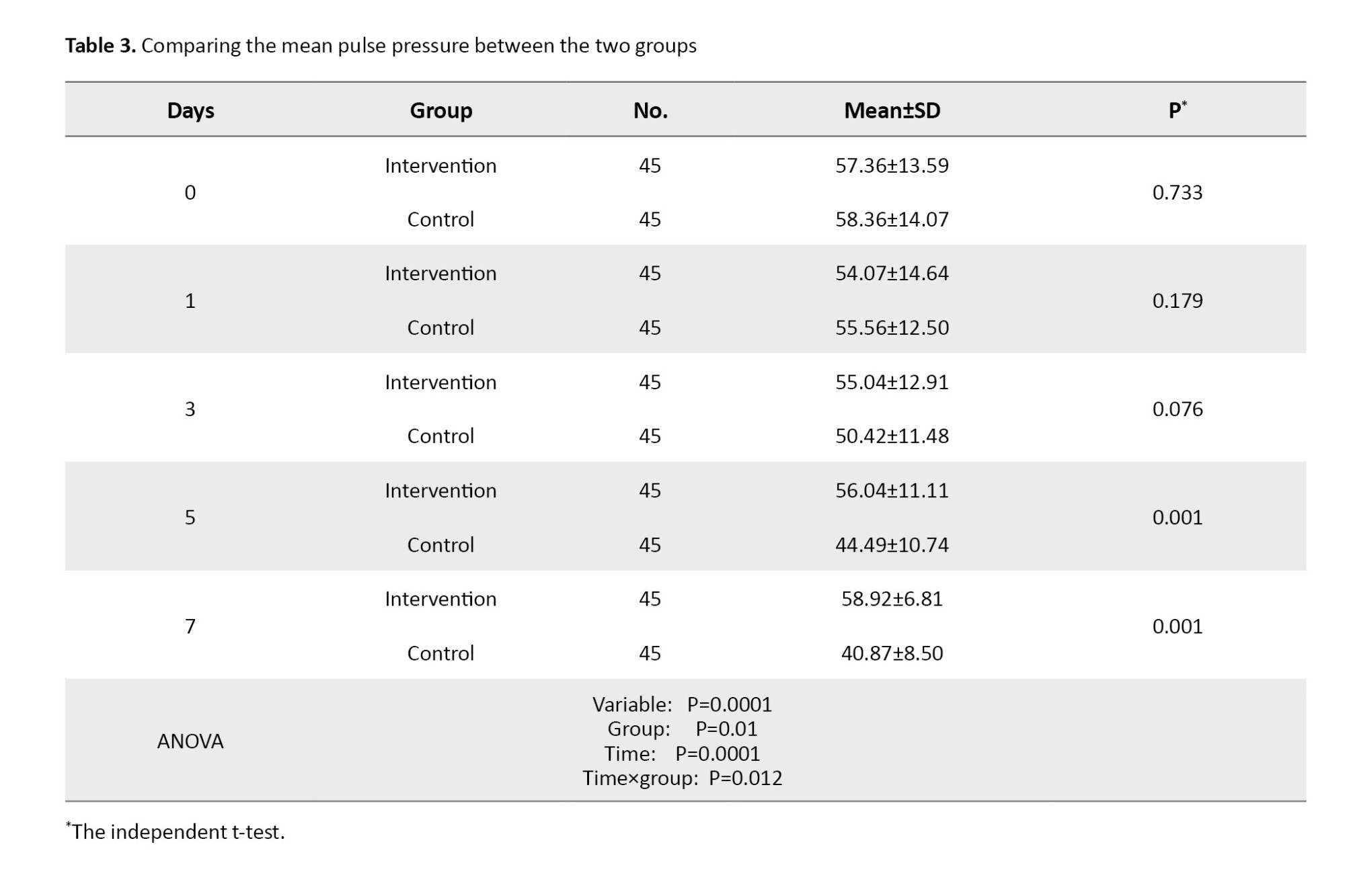
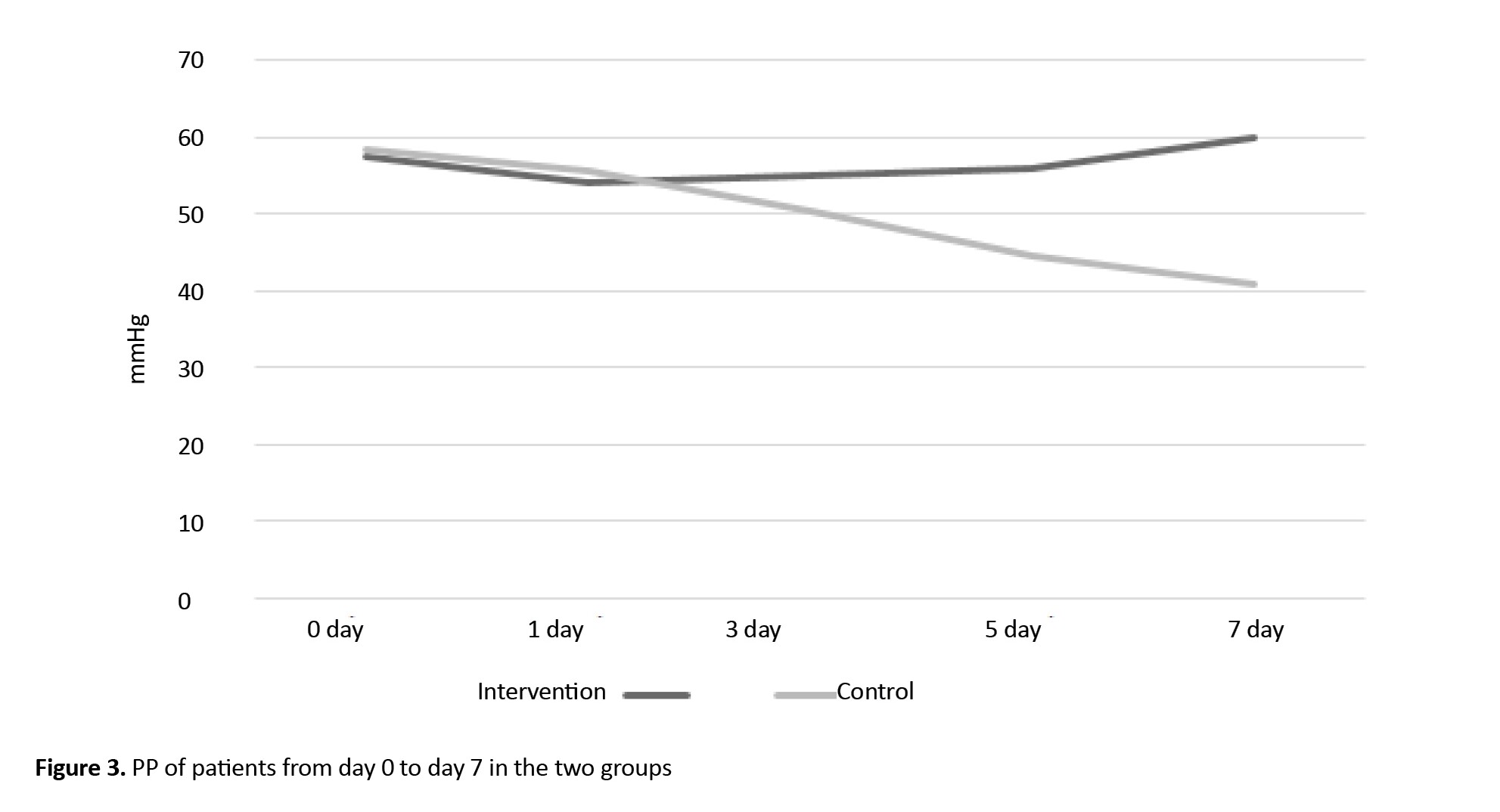
Figure 3 shows the changes in PP over time in two groups.
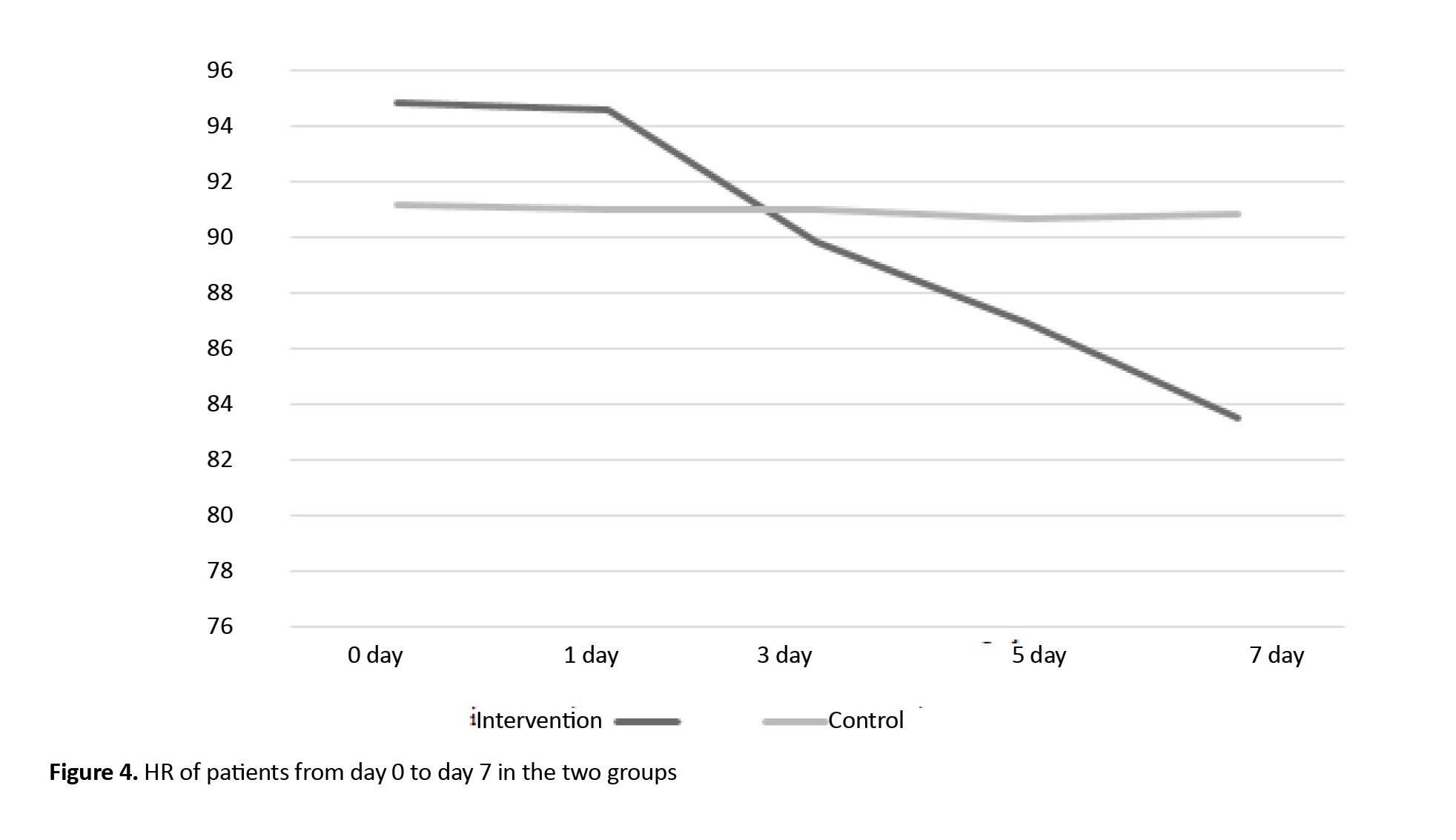
The mean HR values showed no statistically significant difference in the patients of the two study groups before the intervention and during the study (on days 1, 3, 5, and 7). Regarding the intra-group comparison, repeated measure ANOVA showed that HR decreased significantly in the intervention group (P=0.038), but not significantly in the control group. Moreover, time and group had no significant interaction effect on HR. Also, the results of this test showed that the mean HR value was statistically significant between assessment stages (day 0, 3, 5, and 7), as shown in Table 4.
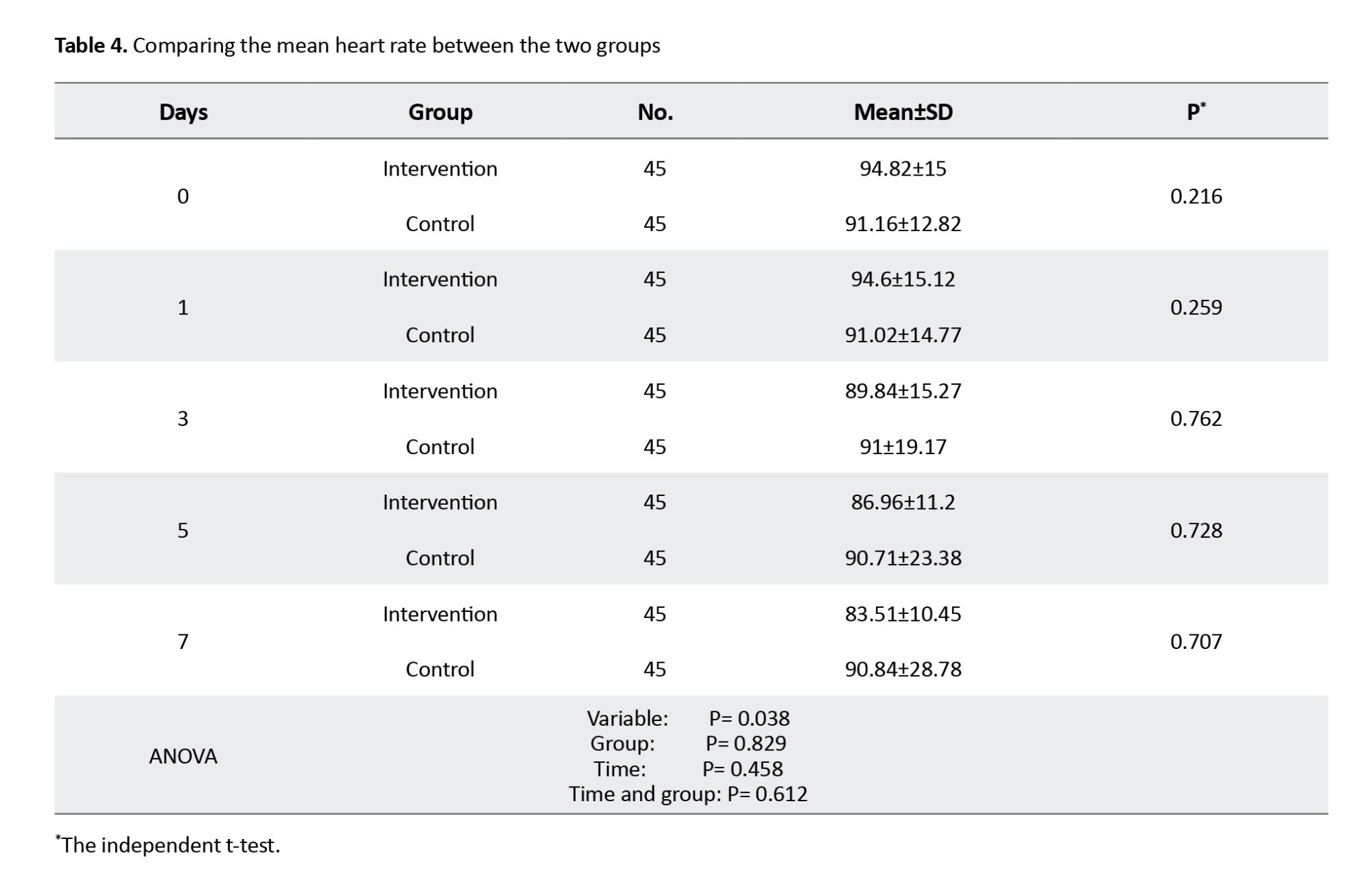
Figure 4 shows the changes in HR over time in the two groups.
Discussion
This study showed that in the intervention group, MAP remained stable from the third day onwards, but in the control group, it decreased significantly from day 3 to 7. It seems that the ABCDE intervention, by reducing the consumption of sedation, making the breathing modes lighter, and moving more, has been able to maintain the MAP of the patients better than the control group and achieve hemodynamic stability. The present study’s findings are consistent with Ren et al. study, which showed that the ABCDE bundle has helped stabilize MAP [3].
This result might be seen because the ABCDE bundle can expand the airway, lower mucus viscosity, and make airway secretions easier to discharge. In addition, ABCDE has been shown to alleviate bronchial spasm and reduce lung tissue inflammation by activating and releasing lipoproteins on the alveolar wall. As a result, the secretion of mucus is allowed to be hydrolyzed, helping to dilute sputum discharge and promoting microcirculation of the lungs [19], improving the ratio of ventilation/blood flow and the circulatory function, enhancing oxygenation index, and improving the patient’s hemodynamics indicators.
After hemodynamics indicators improve, the patient’s cardiopulmonary function increases, and the dose of sedatives and analgesics is reduced. Sedative drugs can inhibit the sympathetic nervous system, leading to decreased angio ectasis and myocardial suppression, which all lead to hemodynamic effects. Therefore, appropriate use of sedatives may stabilize hemodynamics, reduce stress response, lower oxygen consumption, and improve patient comfort and efficacy of the therapeutic strategy [20].
Also, this study’s results showed that the ABCDE bundle helps increase the PP of patients in the intervention group. Negro et al. also obtained similar results [17]. The results of previous studies showed one of the most important hemodynamic changes in patients undergoing mechanical ventilation with positive pressure is an increase in intrathoracic pressure, which leads to a transient decrease in venous return and, subsequently, a reduction in the stroke volume of the right ventricle, and after several heart beats, left ventricular stroke volume decreases [21, 22]. Therefore, mechanical ventilation causes a periodic change in the emptying of the left ventricle, such as a change in the cardiac output. Some of the benefits of the ABCDE bundle are increasing venous return, stroke volume, and cardiac output, ultimately leading to improved PP.
The ABCDE bundle strengthens the intensity of the diaphragm, improves the respiratory state, enhances spontaneous breathing work, and improves circulatory function [11]. These activities suggest that the ABCDE bundle can improve the PP [23, 24].
Also, this study showed no significant difference between the use of the ABCDE bundle and the HR of the patients in the two groups. However, in the intervention group, the heart rate decreased significantly from days 0 to 7. Thus, the ABCDE bundle intervention had reduced HR by maintaining MAP and improving stroke volume. Moreover, mechanical ventilation imposes obvious tensile stress and compressive stress on the alveolar epithelium during the breathing movement [25]. Suppose the duration and intensity of such tensile and compressive stimulation are too great. In that case, these excessive biophysical stimuli might be transformed into the regulatory signals of cytotoxicity and inflammation, leading to cell and tissue damage [26], and the hemodynamics indicators cannot change significantly in the short term.
Of course, in the study of Ren et al., the mean HR of patients in the ABCDE bundle group improved on the seventh day after the intervention compared with the first day [3]. This difference can be attributed to the type of study, the random allocation of patients (sampling at two different times), and the lack of homogeneity of patients in the Ren et al. study. This result has challenged the results of our research, and caution should be taken when generalizing the results. Another reason is the difference in the mean age of the patients. In the Ren et al. study, the mean age of the patients was 61.1 years; in this study, it was 48.2 years. With increasing age, the incidence of tachyarrhythmias increases (in Ren et al. study, the HR of the patients was 109 beats, but in this study, it was 90 beats). A higher HR can be improved more easily with the ABCDE bundle, while in this study, the HR was within the normal range.
One of the limitations of this study was its single center; therefore, results in that context could not be deemed to confirm results from other ICUs. Another limitation of this study is the duration of the intervention, and it is necessary to investigate the long-term effects of the ABCDE bundle. Therefore, it is essential to conduct more extensive multicenter studies to increase the generalizability of the results.
The results of this study showed that the ABCDE bundle helps stabilize MAP and increase PP, but does not positively affect HR. Due to its cheap price and availability, it is suggested that the ICU staff use this valuable strategy to maintain the hemodynamic stability of patients. Of course, more extensive studies are needed to know other benefits and side effects.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.NURSE.REC.1398.024). Also, the study was registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20190625044000N1).
Funding
This study was financially supported by the Research Vice-Chancellor of Mashhad University of Medical Sciences, Mashhad, Iran.
Authors' contributions
Conceptualization and study design: Javad Malekzadeh and Mahsa Ghouchani; Recruiting the study sample s: Mahsa Ghouchani, Mohammad Rajabpour, and Ahmad Bagheri Moghadam; Data analysis: Tahereh Sadeghi and Mohammad Rajabpour; Data interpretation: Javad Malekzadeh, Mohammad Rajabpour, Ahmad Bagheri Moghadam, and Tahereh Sadeghi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all patients, nurses, and officials of Imam Reza Hospital, Mashhad, who cooperated in this study. The authors also thank the financial support of Mashhad University of Medical Sciences, Mashhad, Iran, for implementing the project.
References
Adult patients requiring mechanical ventilation in Intensive Care Units (ICUs) are increasing [1]. Mechanical ventilation improves ventilation, reduces breathing work, strengthens spontaneous breathing, and increases active breathing capacity [2]. Patients under mechanical ventilation undergo sedation to tolerate the endotracheal tube and long-term lying position, prevent fighting with the ventilator, tolerate many procedures, and optimize oxygen consumption [1]. Sedation and analgesia can help increase patient comfort, but it has a non-selective inhibition of blood circulation and breathing centers [3]. Mechanical ventilation may also cause hemodynamic instability in patients [4]. Common adverse cardiovascular responses to mechanical ventilation include barotrauma, lung injury, pneumonia, hemodynamic alterations and instability, myocardial ischemia, autonomic dysfunction, and cardiac dysrhythmias. Other problems include endotracheal tube complications, respiratory muscle weakness, and secretion retention [5].
Examining hemodynamic indicators is an important monitoring tool for critically ill patients. It can not only determine the real response of the body but also help in clinical interventions and appropriate treatment to improve tissue perfusion as soon as possible [6]. Therefore, there is an urgent need to implement interpersonal and evidence-based strategies to reduce the complications associated with long-term mechanical ventilation, long-term sedation, and improving hemodynamic status in adults under mechanical ventilation [7].
The ABCDE bundle (ABC: Awakening, Breathing, and Coordination, D: Delirium and Monitoring, E: Early Mobility) is a multidisciplinary, evidence-based approach that improves the prognosis of patients in the ICU by enhancing the quality of care and outcomes of patients under mechanical ventilation [8]. In the US, the implementation of the ABCDE bundle is supported not only by special care groups but also by national quality improvement organizations as a means to increase the quality and safety of critical care and improve the prognosis of patients [9]. Among the benefits of the ABCDE bundle are reducing the duration of mechanical ventilation, hospitalization, and the prevalence and duration of delirium [10]. Although the previous studies that implemented at least three of the ABCDE bundle components showed positive short-term benefits regarding the length of ICU stay and mortality [9, 10], they reported that bundle implementation did not necessarily reduce the incidence or duration of delirium [11].
The ABCDE bundle is safe and effective [11]. Still, its effects on central venous pressure, Heart Rate (HR), and oxygenation index (PaO2/FiO2) in patients undergoing mechanical ventilation have been reported in a descriptive cross-sectional manner [3]. Also, in previous studies [3, 10, 11], three components of the bundle have mainly been used, and there is limited evidence regarding the effectiveness of the full implementation of this bundle. Besides, its efficacy on the complications of mechanical ventilation has been given less attention, and the results of the studies are contradictory.
Therefore, this study was conducted as a clinical trial to determine the effect of using the ABCDE bundle on the stability of the hemodynamic indicators of patients under mechanical ventilation.
Materials and Methods
This clinical trial was conducted on 90 intubated patients under mechanical ventilation from July to December 2022. The sample size was calculated based on the study of Ren et al. [3]. For this purpose, 45 patients were estimated in each group, taking into account 90% power (1-β), 5% type 1 error (α), effect size of 10, standard deviation of 15, and the correlation between measurements of 0.8. Taking a 10% drop into account, about 100 patients were recruited for the study. The samples were divided into the intervention (n=46) and control groups (n=46) using a random block allocation method (25 block sizes of 4 on the sealed envelope website [12]). One patient in the intervention group and one in the control group were excluded due to not participating in the follow-up (Figure 1). Random concealment was done using the SNOSE (Sequentially Numbered, Opaque, Sealed Envelope) technique.

The inclusion criteria were as follows: Being age 18-60 years old, suffering from subgroups of pulmonary diseases (e.g. chronic obstructive pulmonary diseases, acute respiratory distress syndrome, pneumonia, and embolism) based on medical records, the minimum duration of mechanical ventilation and treatment time of sedation and analgesia ≥48 hours [13], the minimum length of stay in ICU ≥48 hours, no neuromuscular disease (such as Myasthenia Gravis (MG), Muscular-spinal Atrophy (SMA), based on medical record), no physical disorder, Richmond Agitation-sedation Scale (RASS) score ≥0. The exclusion criteria were as follows: Unwillingness to continue participation in research at any stage, patient’s death or transfer from the ICU, and lack of consciousness despite cessation of sedation.
The tools used in the present study were the demographic form, the RASS, the full outline of unresponsiveness (FOUR) score, and the researcher-made checklist of hemodynamic status. The demographic information form included questions about age, gender, marital status, past medical history, and smoking/drug abuse.
The patients’ hemodynamic indicators, including Mean Arterial Pressure (MAP), Pulse Pressure (PP), HR, and Blood Pressure (BP), were measured using Alborz B5 and B9 monitoring devices. The reliability of these devices was checked by regularly calibrating them. Additionally, the research team followed the manufacturer’s guidelines for properly using and maintaining the devices.
The RASS measures the level of restlessness in the ICU. This scale is a 10-point continuum from -5 to +4. The validity of this instrument has been confirmed by Ely and Sessler [14, 15]. The FOUR score scale is an international scale for monitoring the level of consciousness of intubated patients, and the validity and reliability of this tool have already been confirmed [16]. RASS and FOUR scores were measured in both groups of patients before the intervention.
The ABCDE bundle was used in the intervention group. The implementation of the ABCDE bundle was as follows. ABC includes awakening and breathing coordination, and has 4 stages [17]. In the first step, a screening for the Spontaneous Awakening Trial (SAT) safety is done. If the screening is deemed safe, step 2, which involves “doing SAT,” will be carried out, and the administration of sedatives will be discontinued under the supervision of the attending physician.
In the second stage, sedation is resumed with half the previous dose, and start the first stage is started again 24 hours later. If step 2 is successful, step 3 is ignited. The researcher performs the spontaneous breathing trial (SBT) safety screening in this stage according to the protocol. If the failure criteria appear in this stage, stage 1 is repeated 24 hours later.
If step 3 was successful, step 4 is initiated, and “SBT intervention” is performed. Implementing SBT involves discontinuing mechanical ventilation support and using a ventilator with a respiratory rate set at zero, positive end-expiratory pressure/continuous positive airway pressure ≤5, pressure support ventilation ≤5, and a T-tube. This intervention is deemed successful if the patient tolerates spontaneous breathing for 30-120 minutes [18] and the anesthesiologist prescribes extubating. In the event of failure in stage 4, full respiratory support is provided for the patient.
Every morning, the Delirium (D) in patients on mechanical ventilation was assessed using the ICU D assessment diagnostic table. The progression of D was monitored, and the daily assessment scores of patients on mechanical ventilation were recorded. On this basis, the anesthesiologist adjusts the dose of the sedatives and analgesics used. The physicians further identified those diagnosed as positive.
For E stage (early exercise/mobility) and according to the conditions of patients on mechanical ventilation, if they meet the minimum criteria for early mobility, an activity was adopted: Sitting on the edge of the bed, standing by the bed, or sitting in a chair, walking a short distance. During the mobility, the criteria for stopping early mobility were considered according to the protocol [11].
Finally, hemodynamic indicators, including MAP, PP, and HR, were measured at the end of the interventions and after the doctor’s visit on days 0, 1, 3, 5, and 7.
In the control group, according to the routine of the ward, common sedation and pain relief were performed. The patient’s level of consciousness and hemodynamic indicators were measured after the doctor’s visit on days 0, 1, 3, 5, and 7. This process was performed for each patient throughout the week, and depending on the number of active beds in the hospital, 5 to 10 patients were studied each week.
Data analysis was done using SPSS software, version 20, and descriptive statistics, including frequency, Mean±SD, were calculated. Next, quantitative data regarding normal distribution were checked using the Kolmogorov-Smirnov and Shapiro-Wilk tests. The independent t-test was used to compare two groups regarding normal quantitative variables, and the Mann-Whitney U test was used for non-normal quantitative variables (age, FOUR score, and RASS). Qualitative variables (gender, marital status, past medical history, and smoking/drug abuse) were also compared between the two groups using the chi-square test. A repeated-measure ANOVA test was used to compare the intra-groups. In all tests, P<0.05 was considered statistically significant.
Results
Patients’ Mean±SD ages were 48.76±11.46 in the intervention and 47.58±11.56 years in the control group. The result of the Mann-Whitney U test showed no statistically significant difference between the two groups in this respect. Also, the Mean±SD of the FOUR score scales were 7.78±1.24 in the intervention group patients and 8.00±1.10 in the control group. The result of the Mann-Whitney test showed no statistically significant difference between the two groups in this respect. The Mean±SD scores of the RASS scale were -2.16±0.71 in the intervention and -2.00±0.77 in the control group. The result of the Mann-Whitney U test showed no statistically significant difference between the two groups in this regard. The other demographic and disease characteristics of the two groups are listed in Table 1.

The result of the independent t-test showed that the mean MAP before the intervention (day 0) and the first day was not statistically significantly different in the two study groups. However, the Mean±SD MAP were 79.39±10.9 on the day 3, 74.2±13.6 on the day 5, and 68.7±14.8 on the day 7 in the control group, which showed a statistically significant decrease compared to the intervention group (P<0.05). Also, the repeated measures ANOVA results showed that the effects of time, group, and time×group on the mean MAP were statistically significant (P=0.0001). The post hoc (Bonferroni) test revealed that the mean MAP from day 3 to day 7 significantly decreased (P=0.0001). These results were shown in Table 2.

Figure 2 shows the changes in MAP over time in two groups.

The mean PP values showed no statistically significant difference before the intervention (day 0) and on days 1 to 3 in the patients of the two groups. However, the Mean±SD PP values were 56.04±11.11 on the day 5 and 58.92±6.81 on the day 7 in the intervention group. The independent t-test results showed that in the intervention group, from the 5 to the day 7, the mean PP value increased significantly compared to the control group (P=0.0001). The results of the repeated measures ANOVA demonstrated statistically significant effects of time, group, and time×group on the mean PP (P=0.012). Also, post hoc test (Bonferroni test) indicated a significant decrease in the mean PP value from day 5 to day 7 (P=0.001). These results were shown in Table 3.

Figure 3 shows the changes in PP over time in two groups.

The mean HR values showed no statistically significant difference in the patients of the two study groups before the intervention and during the study (on days 1, 3, 5, and 7). Regarding the intra-group comparison, repeated measure ANOVA showed that HR decreased significantly in the intervention group (P=0.038), but not significantly in the control group. Moreover, time and group had no significant interaction effect on HR. Also, the results of this test showed that the mean HR value was statistically significant between assessment stages (day 0, 3, 5, and 7), as shown in Table 4.

Figure 4 shows the changes in HR over time in the two groups.

Discussion
This study showed that in the intervention group, MAP remained stable from the third day onwards, but in the control group, it decreased significantly from day 3 to 7. It seems that the ABCDE intervention, by reducing the consumption of sedation, making the breathing modes lighter, and moving more, has been able to maintain the MAP of the patients better than the control group and achieve hemodynamic stability. The present study’s findings are consistent with Ren et al. study, which showed that the ABCDE bundle has helped stabilize MAP [3].
This result might be seen because the ABCDE bundle can expand the airway, lower mucus viscosity, and make airway secretions easier to discharge. In addition, ABCDE has been shown to alleviate bronchial spasm and reduce lung tissue inflammation by activating and releasing lipoproteins on the alveolar wall. As a result, the secretion of mucus is allowed to be hydrolyzed, helping to dilute sputum discharge and promoting microcirculation of the lungs [19], improving the ratio of ventilation/blood flow and the circulatory function, enhancing oxygenation index, and improving the patient’s hemodynamics indicators.
After hemodynamics indicators improve, the patient’s cardiopulmonary function increases, and the dose of sedatives and analgesics is reduced. Sedative drugs can inhibit the sympathetic nervous system, leading to decreased angio ectasis and myocardial suppression, which all lead to hemodynamic effects. Therefore, appropriate use of sedatives may stabilize hemodynamics, reduce stress response, lower oxygen consumption, and improve patient comfort and efficacy of the therapeutic strategy [20].
Also, this study’s results showed that the ABCDE bundle helps increase the PP of patients in the intervention group. Negro et al. also obtained similar results [17]. The results of previous studies showed one of the most important hemodynamic changes in patients undergoing mechanical ventilation with positive pressure is an increase in intrathoracic pressure, which leads to a transient decrease in venous return and, subsequently, a reduction in the stroke volume of the right ventricle, and after several heart beats, left ventricular stroke volume decreases [21, 22]. Therefore, mechanical ventilation causes a periodic change in the emptying of the left ventricle, such as a change in the cardiac output. Some of the benefits of the ABCDE bundle are increasing venous return, stroke volume, and cardiac output, ultimately leading to improved PP.
The ABCDE bundle strengthens the intensity of the diaphragm, improves the respiratory state, enhances spontaneous breathing work, and improves circulatory function [11]. These activities suggest that the ABCDE bundle can improve the PP [23, 24].
Also, this study showed no significant difference between the use of the ABCDE bundle and the HR of the patients in the two groups. However, in the intervention group, the heart rate decreased significantly from days 0 to 7. Thus, the ABCDE bundle intervention had reduced HR by maintaining MAP and improving stroke volume. Moreover, mechanical ventilation imposes obvious tensile stress and compressive stress on the alveolar epithelium during the breathing movement [25]. Suppose the duration and intensity of such tensile and compressive stimulation are too great. In that case, these excessive biophysical stimuli might be transformed into the regulatory signals of cytotoxicity and inflammation, leading to cell and tissue damage [26], and the hemodynamics indicators cannot change significantly in the short term.
Of course, in the study of Ren et al., the mean HR of patients in the ABCDE bundle group improved on the seventh day after the intervention compared with the first day [3]. This difference can be attributed to the type of study, the random allocation of patients (sampling at two different times), and the lack of homogeneity of patients in the Ren et al. study. This result has challenged the results of our research, and caution should be taken when generalizing the results. Another reason is the difference in the mean age of the patients. In the Ren et al. study, the mean age of the patients was 61.1 years; in this study, it was 48.2 years. With increasing age, the incidence of tachyarrhythmias increases (in Ren et al. study, the HR of the patients was 109 beats, but in this study, it was 90 beats). A higher HR can be improved more easily with the ABCDE bundle, while in this study, the HR was within the normal range.
One of the limitations of this study was its single center; therefore, results in that context could not be deemed to confirm results from other ICUs. Another limitation of this study is the duration of the intervention, and it is necessary to investigate the long-term effects of the ABCDE bundle. Therefore, it is essential to conduct more extensive multicenter studies to increase the generalizability of the results.
The results of this study showed that the ABCDE bundle helps stabilize MAP and increase PP, but does not positively affect HR. Due to its cheap price and availability, it is suggested that the ICU staff use this valuable strategy to maintain the hemodynamic stability of patients. Of course, more extensive studies are needed to know other benefits and side effects.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.NURSE.REC.1398.024). Also, the study was registered by the Iranian Registry of Clinical Trials (IRCT), Tehran, Iran (Code: IRCT20190625044000N1).
Funding
This study was financially supported by the Research Vice-Chancellor of Mashhad University of Medical Sciences, Mashhad, Iran.
Authors' contributions
Conceptualization and study design: Javad Malekzadeh and Mahsa Ghouchani; Recruiting the study sample s: Mahsa Ghouchani, Mohammad Rajabpour, and Ahmad Bagheri Moghadam; Data analysis: Tahereh Sadeghi and Mohammad Rajabpour; Data interpretation: Javad Malekzadeh, Mohammad Rajabpour, Ahmad Bagheri Moghadam, and Tahereh Sadeghi; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all patients, nurses, and officials of Imam Reza Hospital, Mashhad, who cooperated in this study. The authors also thank the financial support of Mashhad University of Medical Sciences, Mashhad, Iran, for implementing the project.
References
- Smith HAB, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A, et al. 2022 Society of critical care medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically Ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med. 2022; 23(2):e74-110. [DOI:10.1097/PCC.0000000000002873] [PMID]
- Wang H, Wang C, Wang Y, Tong H, Feng Y, Li M, et al. Sedative drugs used for mechanically ventilated patients in intensive care units: A systematic review and network meta-analysis. Curr Med Res Opin. 2019; 35(3):435-46. [DOI:10.1080/03007995.2018.1509573] [PMID]
- Ren XL, Li JH, Peng C, Chen H, Wang HX, Wei XL, et al. Effects of ABCDE bundle on hemodynamics in patients on mechanical ventilation. Med Sci Monit. 2017; 23:4650-6. [DOI:10.12659/MSM.902872] [PMID]
- Amini S, Morovatdar N, Karrari SP, Asadpour A, Abbasi Tashnizi M, Moeinipoor AA, et al. The risk factors of prolonged mechanical ventilation after isolated coronary artery bypass graft surgery. EvidBased Care. 2023; 13(1):7-14. [Link]
- Grübler MR, Wigger O, Berger D, Blöchlinger S. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly. 2017; 147:w14491. [DOI:10.4414/smw.2017.14491] [PMID]
- Hussein K, Ahmed AF, Omar MMA, Galhom RA, Salah M, Elrouby O, et al. Assessment of hemodynamics, blood gases, and lung histopathology of healthy Pig model on two different mechanical ventilators. Heliyon. 2022; 8(9):e10736. [DOI:10.1016/j.heliyon.2022.e10736] [PMID]
- Jubran A, Grant BJB, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, et al. Long-term outcome after prolonged mechanical ventilation. A long-term acute-care hospital study. Am J Respir Crit Care Med. 2019; 199(12):1508-16. [DOI:10.1164/rccm.201806-1131OC] [PMID]
- Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF Bundle in critical care. Crit Care Clin. 2017; 33(2):225-43. [DOI:10.1016/j.ccc.2016.12.005] [PMID]
- Moraes FDS, Marengo LL, Moura MDG, Bergamaschi CC, de Sá Del Fiol F, Lopes LC, et al. ABCDE and ABCDEF care bundles: A systematic review of the implementation process in intensive care units. Medicine (Baltimore). 2022; 101(25):e29499. [DOI:10.1097/MD.0000000000029499] [PMID]
- Otusanya OT, Hsieh SJ, Gong MN, Gershengorn HB. Impact of ABCDE bundle implementation in the intensive care unit on specific patient costs. J Intensive Care Med. 2022; 37(6):833-41. [DOI:10.1177/08850666211031813] [PMID]
- Sosnowski K, Lin F, Chaboyer W, Ranse K, Heffernan A, Mitchell M. The effect of the ABCDE/ABCDEF bundle on delirium, functional outcomes, and quality of life in critically ill patients: A systematic review and meta-analysis. Int J Nurs Stud. 2023; 138:104410. [DOI:10.1016/j.ijnurstu.2022.104410] [PMID]
- Singh J. Randomization and online databases for clinical trials. J Pharmacol Pharmacother. 2014; 5(2):173-4. [DOI: 10.4103/0976-500X.130155] [PMID]
- Sosnowski K, Mitchell M, Cooke M, White H, Morrison L, Lin F. Effectiveness of the ABCDEF bundle on delirium, functional outcomes and quality of life in intensive care patients: A study protocol for a randomised controlled trial with embedded process evaluation. BMJ Open. 2021; 11(7):e044814. [DOI:10.1136/bmjopen-2020-044814] [PMID]
- Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003; 289(22):2983-91. [DOI:10.1001/jama.289.22.2983] [PMID]
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166(10):1338-44. [DOI:10.1164/rccm.2107138] [PMID]
- Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Ann Neurol. 2005; 58(4):585-93. [DOI:10.1002/ana.20611] [PMID]
- Negro A, Bambi S, De Vecchi M, Isotti P, Villa G, Miconi L, et al. The ABCDE bundle implementation in an intensive care unit: Facilitators and barriers perceived by nurses and doctors. Int J Nurs Pract. 2022; 28(2):e12984. [DOI:10.1111/ijn.12984] [PMID]
- Erbay Dalli Ö, Akça Doğan D, Bayram R, Pehlivan S, Yildiz H. Practices of the ABCDEF care bundle in intensive care units as reported by nurses: A cross-sectional study from Turkey. Nurs Crit Care. 2024; 29(5):974-86. [DOI: 10.1111/nicc.12963] [PMID]
- Ding R, Zhao D, Li X, Liu B, Ma X. Rho-kinase inhibitor treatment prevents pulmonary inflammation and coagulation in lipopolysaccharide-induced lung injury. Thromb Res. 2017; 150:59-64. [PMID]
- Wang J, Yang YD, She QF, Tang Y. Effect of sequential assist-control ventilation on cardio-pulmonary function and systemic inflammatory state of chronic pulmonary heart disease complicated with respiratory failure patients. J Hainan Med Univ. 2018; 24(8):10-3. [Link]
- Taccheri T, Gavelli F, Teboul JL, Shi R, Monnet X. Do changes in pulse pressure variation and inferior vena cava distensibility during passive leg raising and tidal volume challenge detect preload responsiveness in case of low tidal volume ventilation? Crit Care. 2021; 25(1):110. [DOI:10.1186/s13054-021-03515-7] [PMID]
- Teboul JL, Monnet X, Chemla D, Michard F. Arterial pulse pressure variation with mechanical ventilation. Am J Respir Crit Care Med. 2019; 199(1):22-31. [DOI:10.1164/rccm.201801-0088CI] [PMID]
- Clarissa C, Salisbury L, Rodgers S, Kean S. Early mobilisation in mechanically ventilated patients: A systematic integrative review of definitions and activities. J Intensive Care. 2019; 7:3. [DOI:10.1186/s40560-018-0355-z] [PMID]
- Prohaska CC, Sottile PD, Nordon-Craft A, Gallagher MD, Burnham EL, Clark BJ, et al. Patterns of utilization and effects of hospital-specific factors on physical, occupational, and speech therapy for critically ill patients with acute respiratory failure in the USA: Results of a 5-year sample. Crit Care. 2019; 23(1):175. [DOI:10.1186/s13054-019-2467-9] [PMID]
- Katira BH. Ventilator-induced lung injury: Classic and novel concepts. Respir Care. 2019; 64(6):629-37. [DOI:10.4187/respcare.07055] [PMID]
- Marini JJ, Rocco PRM, Gattinoni L. Static and dynamic contributors to ventilator-induced lung injury in clinical practice. Pressure, Energy, and Power. Am J Respir Crit Care Med. 2020; 201(7):767-74. [DOI:10.1164/rccm.201908-1545CI] [PMID]
Article Type : Research |
Subject:
Special
Received: 2023/12/12 | Accepted: 2025/05/12 | Published: 2025/06/10
Received: 2023/12/12 | Accepted: 2025/05/12 | Published: 2025/06/10
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



