Fri, Jan 30, 2026
Volume 35, Issue 4 (9-2025)
JHNM 2025, 35(4): 307-314 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adi Suryawan I P, Dahlia D, Ayu Kurnia D. Effect of Home-Based Foot-ankle Exercises on Ankle-brachial Index value in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JHNM 2025; 35 (4) :307-314
URL: http://hnmj.gums.ac.ir/article-1-2134-en.html
URL: http://hnmj.gums.ac.ir/article-1-2134-en.html
1- Nursing Science (MSc), Department of Medical Surgical Nursing, Faculty of Nursing, Universitas Indonesia, Depok, Indonesia.
2- Assistant Professor, Department of Medical Surgical Nursing, Faculty of Nursing, Universitas Indonesia, Depok, Indonesia. ,debie@ui.ac.id
3- Instructor, Department of Medical Surgical Nursing, Faculty of Nursing, Universitas Indonesia, Depok, Indonesia.
2- Assistant Professor, Department of Medical Surgical Nursing, Faculty of Nursing, Universitas Indonesia, Depok, Indonesia. ,
3- Instructor, Department of Medical Surgical Nursing, Faculty of Nursing, Universitas Indonesia, Depok, Indonesia.
Full-Text [PDF 569 kb]
(244 Downloads)
| Abstract (HTML) (462 Views)
Full-Text: (155 Views)
Introduction
uncontrolled blood glucose is a major risk factor for complications in patients with Diabetes Mellitus (DM), including those with Peripheral Arterial Disease (PAD) and Diabetic Foot Ulcers (DFUs). Physical exercise is a key non-pharmacological therapy to improve glycemic control and peripheral circulation [1, 2]. During the COVID-19 pandemic, reduced physical activity among DM patients further heightened the risk of foot complications, drawing attention to home-based interventions [3, 4]. Globally, the prevalence of DM is rising rapidly, projected to reach 21.3 million by 2030 [5]. In Indonesia, the national DM prevalence increased from 6.9% in 2013 to 8.5% in 2018, with Denpasar in Bali reporting the highest rate (2%) [6]. PAD affected approximately 236 million individuals aged ≥40 years worldwide in 2019, predominantly in low-income countries [7]. In Indonesia, the incidence rate is 13,807 per one million population [6]. PAD in DM patients, if unaddressed, leads to reduced Ankle Brachial Index (ABI) values and a higher risk of DFUs [8, 9].
Standard diabetic foot exercises, commonly promoted in health services, focus primarily on ankle movements and lack components needed to improve circulation throughout the foot. In contrast, Home-Based Foot-Ankle Exercise (HBFAE) targets all foot and ankle muscles [10, 11]. The difference between the HBFAE and the standard diabetic foot exercise is that the latter consists of only two exercise categories, namely stretching and strengthening exercises, which are further divided into five types of exercise, whereas the former consists of four exercise categories, namely stretching, strengthening, resistance, and balance exercises, which are further divided into ten types of exercise. This is in line with Colberg et al.’s recommendations, emphasizing the combination of aerobic, resistance, and flexibility/balance exercises for optimal outcomes [12]. A preliminary study revealed that, although the patients regularly performed standard foot exercises, many continued to experience foot complaints such as numbness and tingling. Respondents expressed the need for more effective exercise methods that could strengthen the calf, foot, and ankle muscles [13, 14]. A study suggested that resistance training may positively affect both vascular and neurological outcomes in DM patients [15]. Resistance training can significantly improve muscle strength, dynamic balance, and physical function in DM patients with comorbid knee osteoarthritis [16].
The results regarding the HBFAE effects are contradictory. For example, Silva et al. reported that an 8-week HBFAE did not significantly reduce modifiable DFU risk factors, though it helped maintain gait stability over 16 weeks [13]. Given these discrepancies, further investigation is needed to assess the potential benefits of HBFAE, particularly regarding its impact on the ABI value. Therefore, this study aims to evaluate the effectiveness of HBFAE in improving ABI values of patients with Type 2 DM (T2DM).
Materials and Methods
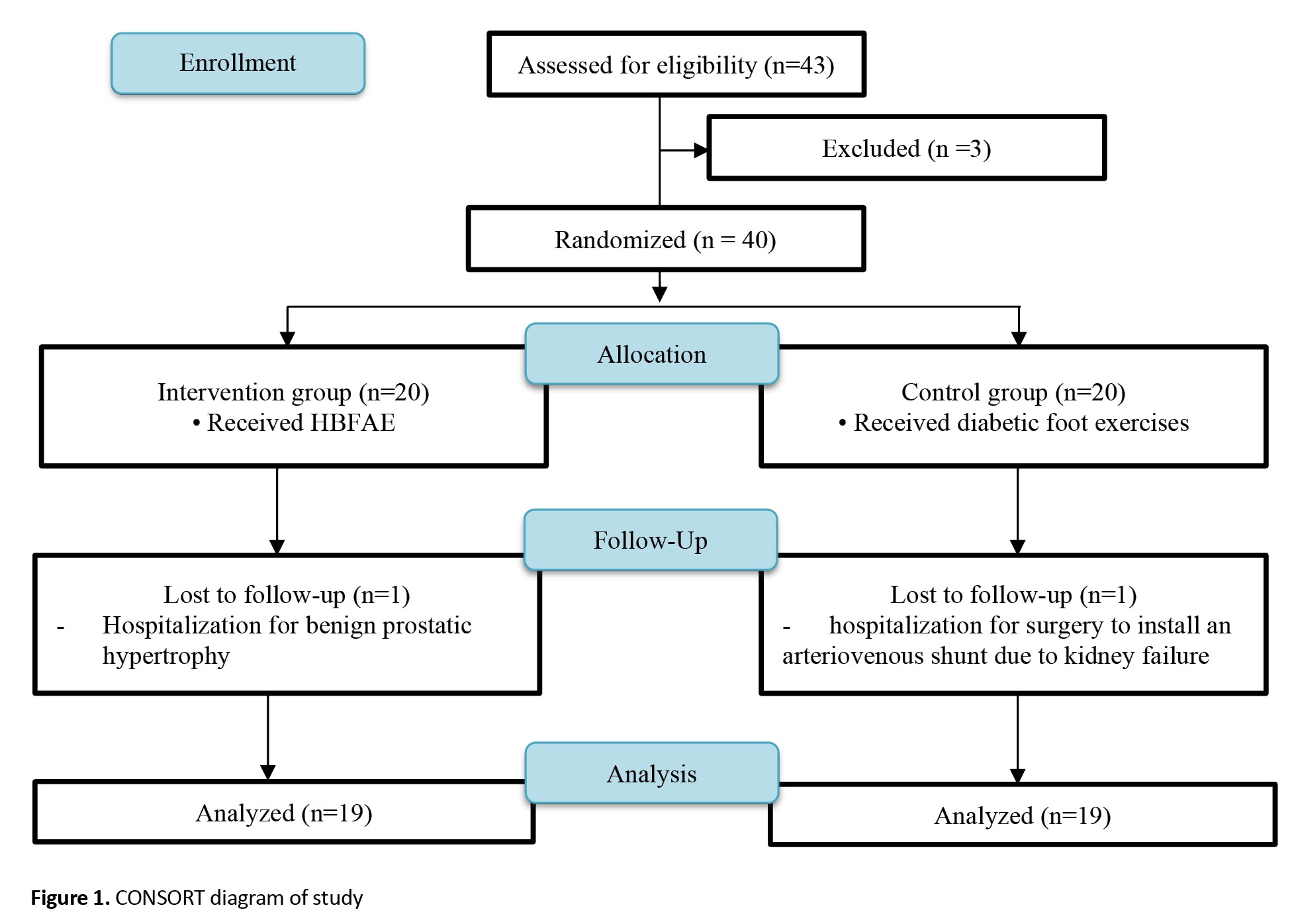
This is a randomized clinical trial that follows the CONSORT 2010 guidelines [17]. Participants were 40 patients with T2DM who visited the Diabetes Polyclinic in Denpasar and Badung Regency, Indonesia, for diabetes control. They were included in the study after signing an informed consent form and meeting the following inclusion criteria: Having an ABI value <0.9 in one or both extremities, experiencing one or more peripheral perfusion complaints in the foot (such as tingling, numbness, burning sensation, and feeling as if wearing socks), ability to walk without assistance for at least 10 m, age 40-65 years, having at most one amputated toe (other than the hallux), residency in Denpasar and Badung Regency, Indonesia (study location), and having at least one family member willing to supervise the exercise intervention. The exclusion criteria were having an active plantar ulcer (diabetic foot) and a history of surgery to the knee, ankle, or hip. Participants were then randomly assigned to equal groups: 20 in the intervention group and 20 in the control group. Randomization was carried out by an individual independent of the research team using the block randomization method. Each block contained four subjects, resulting in six variations of blocks. Both the researchers and participants were unaware of the group allocation. The computer-generated random numbers were placed in an opaque envelope and assigned based on the respondent’s arrival sequence number. Two participants left the study. Finally, 38 participants were assessed: 19 in the intervention group and 19 in the control group (Figure 1). To estimate the sample size, we used the following parameters adapted from a similar study conducted by Barone Gibbs et al. [18]. The standard deviation of the intervention and control groups was considered 0.14 and 0.02, respectively. The combined standard deviation of both groups after calculation was 0.095, and the mean difference between the intervention and control groups was 0.115. Type I error was set at 5% (two-tailed hypothesis), resulting in Zα=1.96, and Type II error was set at 10%, resulting in Zβ=1.645, considering a test power of 80%. The sample size was then estimated to be 36 participants, and 10% was added to account for potential dropouts, resulting in an estimated sample size of 40.
Three instruments were used in this research: a questionnaire surveying the respondent’s characteristics, including gender, smoking history, and hypertension history, a calibrated glucometer for blood glucose measurement, and a calibrated sphygmomanometer and 8-MHz portable Doppler probes for ABI measurement. The sphygmomanometer was calibrated by comparing its measurements with those of a verified standard mercury sphygmomanometer, performed by trained personnel. The 8 MHz portable Doppler was calibrated by checking the sound quality and accuracy of pressure readings, ensuring that the device could clearly detect blood flow. Recalibration was performed weekly during the data collection period to maintain measurement consistency. The assessors, who were not involved in delivering the intervention, conducted the pre-test and post-test ABI assessments. The assessors were blinded to the group allocation. They were previously trained on how to measure ABI, and their inter-rater agreement was assessed using the kappa test (kappa value=0.874). The ABI measurement was taken one day before the intervention (pre-test) and one day after the 24th intervention (post-test). Researchers checked blood sugar twice a week before exercise and when there were signs & symptoms of hypoglycemia in patients (they had previous education about the signs and symptoms of hypoglycemia, and its prevention and treatment methods). For those with blood glucose levels <100 mg/dL, training was delayed, and hypoglycemic therapy was administered first.
The intervention group was asked to perform the HBFAEs, including four exercises divided into ten types. These exercises included foot/ankle stretching and ankle range-of-motion exercises, foot/ankle strengthening, foot/ankle resistance training, and balance exercises. The HBFEAs were performed once a day, 10 movements of 3-5 minutes each day (total time=30-50 minutes per exercise). The control group performed the standard diabetic foot exercise, which consisted of seven types of exercises. Both groups underwent treatment five times a week for 24 sessions (5 weeks). The researchers monitored the implementation of the HBFEAs and diabetic foot exercises by visiting the respondents’ houses twice a week, or through videocalling a family member, three times a week. Two co-researchers who already had a good clinical practice certificate assisted the researchers. Research assistants assisted the main researcher in training, supervising, and conducting home visits to observe the implementation of the HBFEA and diabetic foot exercises. The level of agreement between the researchers and assistants regarding the implementation of the HBFEA and diabetic foot exercises was tested using the Kappa test. The kappa coefficient value obtained for the agreement between the main researcher and assistant 1 was 0.794 (>0.6), and the kappa coefficient value between the main researcher and assistant 2 was 0.659 (>0.6).
The statistical analysis was performed in SPSS software, version 23. frequency and Mean±SD were used to describe the data. Furthermore, differences in the ABI values after treatment between the control and intervention groups were determined using an independent t-test. Differences in ABI values within groups were analyzed using a paired t-test. P<0.05 was considered statistically significant. The data for the normal distribution were tested using the Shapiro-Wilk test. The N-gain score was calculated to assess the effectiveness of therapy based on the ideal ABI value. The multivariate regression analysis was used to find the variables that can predict the changes in ABI values.
Results
During the research process, none of the respondents experienced injuries or complications from the exercise intervention. The demographic/clinical characteristics of the participants, including gender, smoking history, history of foot ulcers, and history of hypertension, are shown in Table 1.
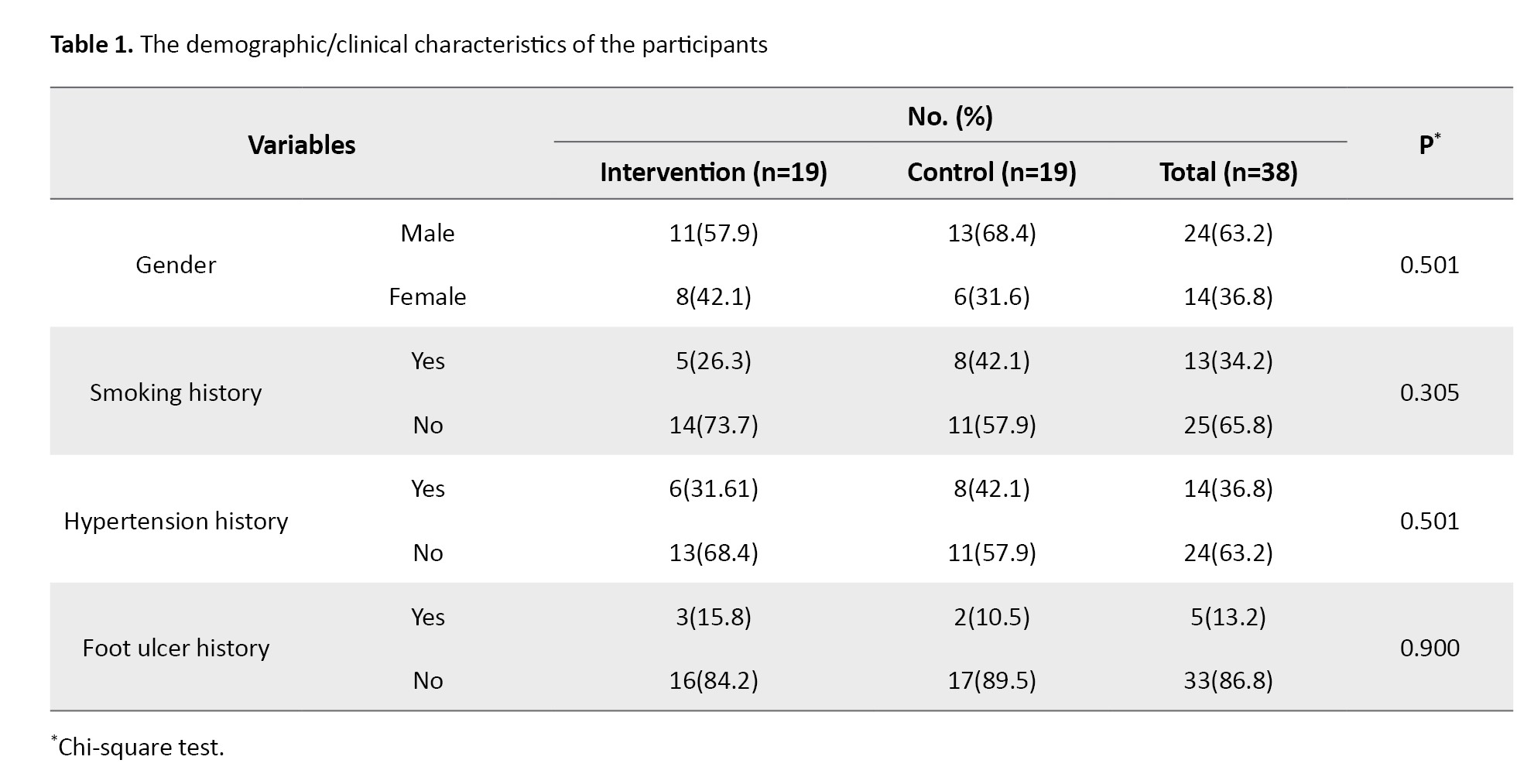
The majority of respondents in both groups were male (57.9% in the intervention group and 68.4% in the control group), with no smoking history (73.7% in the intervention group and 57.9% in the control group), with no history of hypertension (68.4% in the intervention group and 57.9% in the control group), and with no history of foot ulcers (84.2% in the intervention group and 89.5% in the control group). The results of the chi-square test showed no significant differences between the two groups in terms of gender, smoking history, hypertension history, and history of foot ulcers. Other respondent characteristics, including random blood glucose levels and DM disease duration, are presented in Table 2.
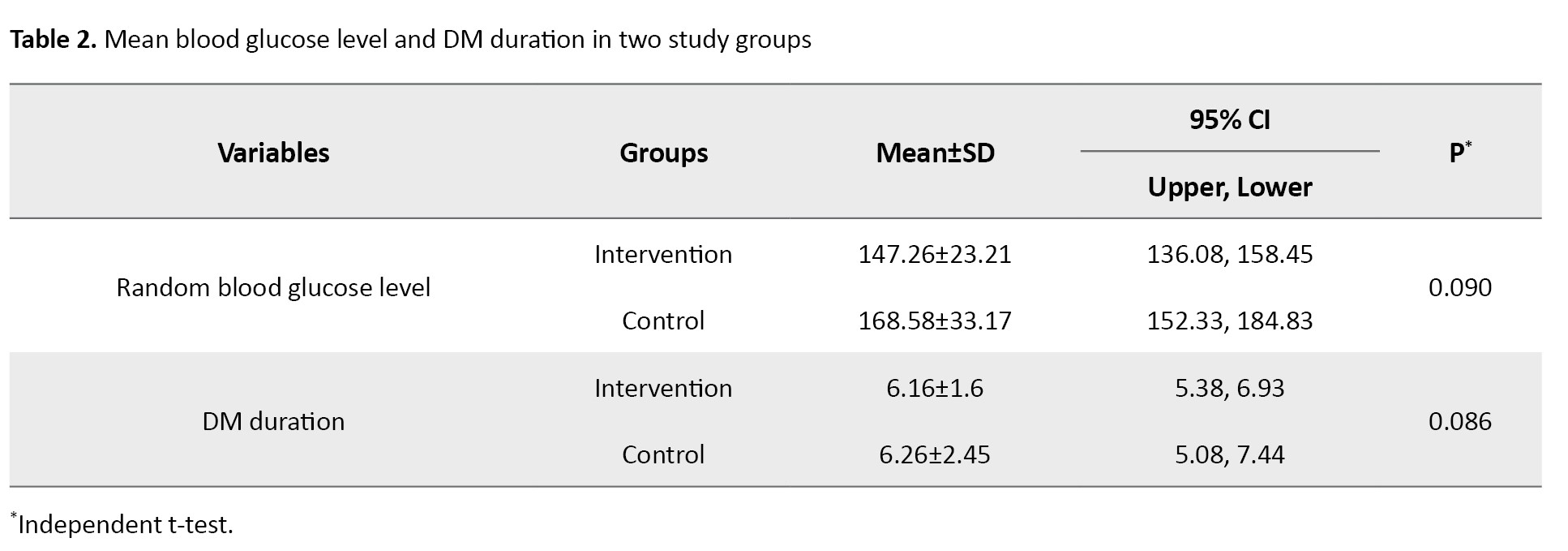
The mean blood glucose level was 147±23.21 mg/dL in the intervention group and 168±33.17 mg/dL in the control group; and had been diagnosed with diabetes for 6 years (intervention and control group).
The mean ABI values for the intervention and control groups are presented in Table 3.
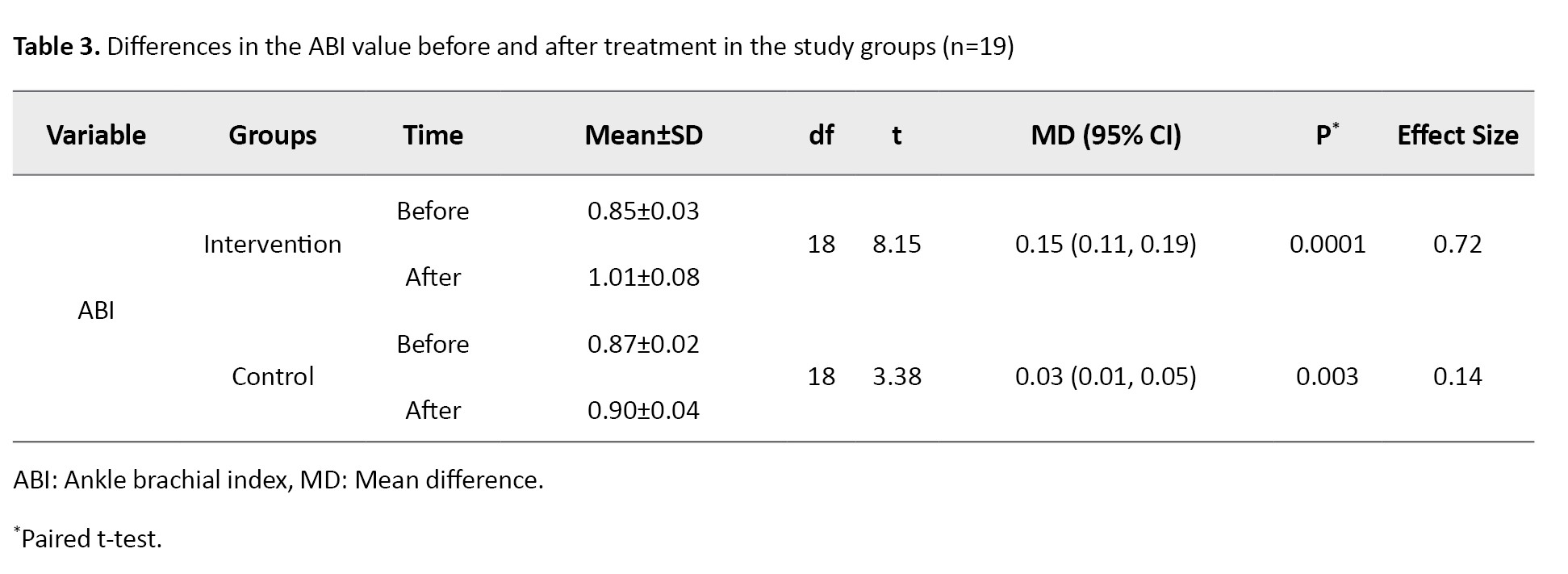
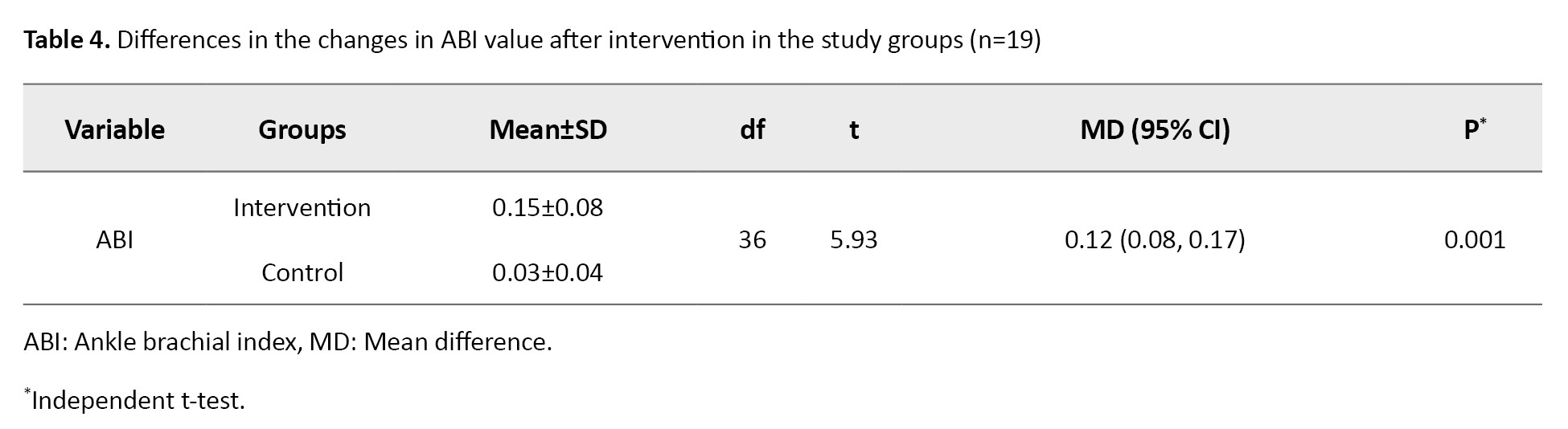
The results of the independent t-test indicated that both therapies increased the ABI value significantly (P<0.05), where the difference in mean ABI value in the intervention group was greater than that in the control group (0.15 vs 0.03). In the N-gain score test, the ideal ABI value was determined to be 1.1. According to the N-gain score test results, the HBFAE had an effectiveness score (effect size) of 0.72 (72%), which suggests that the therapy was highly effective (g>0.7), whereas the diabetic foot exercise had an effectiveness score (effect size) of 0.14, indicating that the standard diabetic foot exercise was not effective (g<0.3). The mean difference in ABI value between the intervention and control groups after intervention was significant (Mean difference=0.12, 95% CI; 0.08%, 0.17%, P=0.001), as shown in Table 4.
The results of multivariate regression analysis are presented in Table 5.
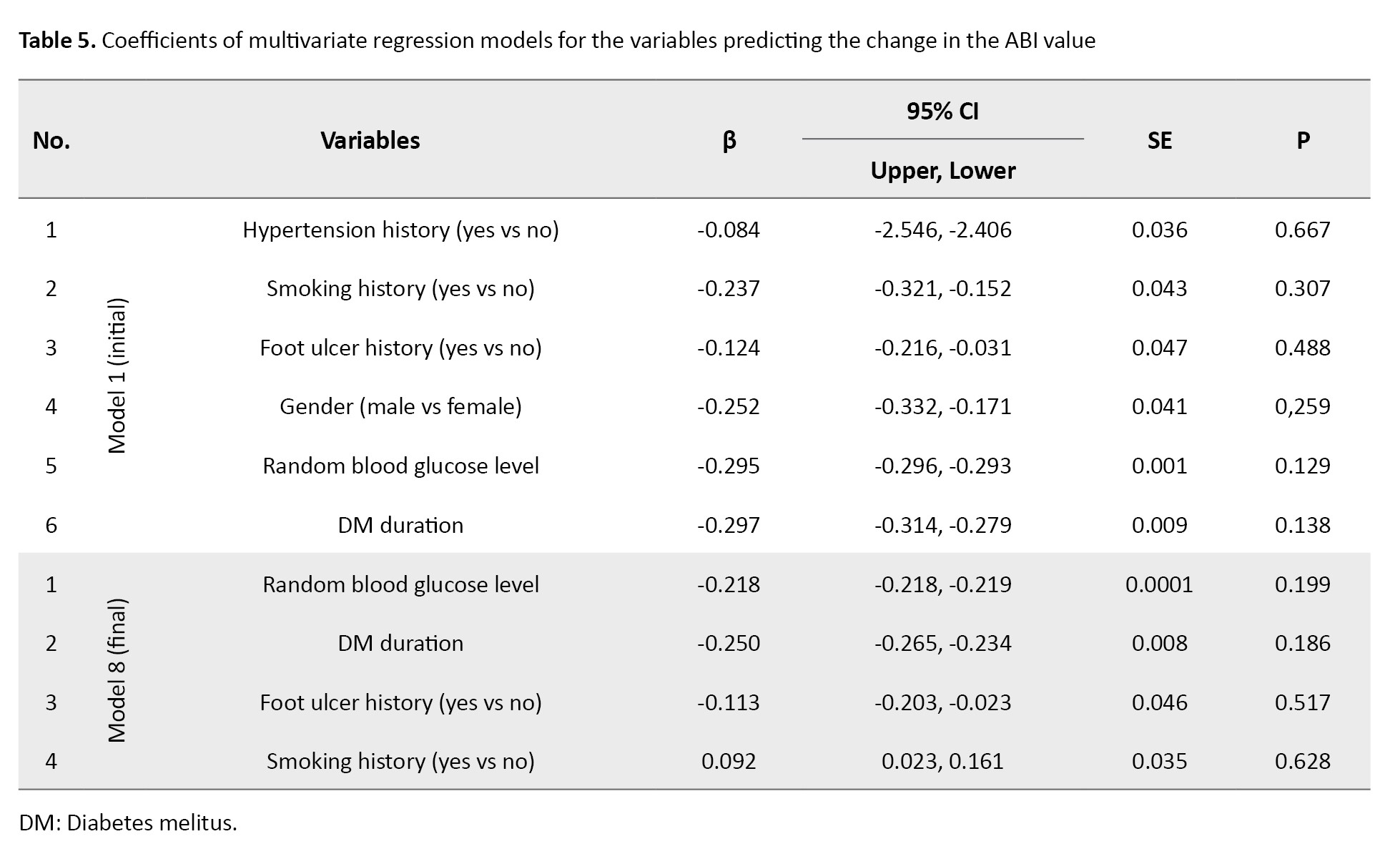
In the final regression model, four confounding variables with a difference >10% in OR during the variable selection process were included. The results showed these four variables (random blood glucose level, DM duration, foot ulcer history, and smoking history) did not have any significant association with the ABI value (P>0.05).
Discussion
This study found that the HBFAE was more effective than the standard diabetic foot exercise in increasing the ABI value. The greater effectiveness of HBFAE can be attributed to its comprehensive structure, which includes stretching, strengthening, resistance, and balance exercises. These components target all foot and ankle muscles, enhancing both local and systemic vascularization [13, 19]. Several studies have also reported the benefits of resistance and balance exercises in improving muscle strength, foot function, and circulation. According to Chen et al. resistance training with TheraBand could increase muscle strength, dynamic balance, and physical function, but its effect on the ABI value was not evaluated [16]. Gholami et al. demonstrated that a resistance-based program significantly improved the ABI values [15]. Similarly, Silva et al. reported improvements in proprioception, peripheral nerve sensitivity, and gait through balance exercises. However, although an 8-week HBFAE maintained gait stability, it did not significantly reduce modifiable risk factors for DFU [13]. Li et al. found that combining the ABI with the percentage of mean arterial pressure at the ankle significantly improved the prediction of all-cause mortality in patients with T2DM compared to using the ABI alone [20].
Resistance training promotes neurovascular recovery by increasing Schwann cell proliferation and neurotrophin expression, while balance exercises improve foot structure, motor neuropathy, and venous return. These combined effects may explain the enhanced ABI scores after HBFAE in our study. Furthermore, the HBFAE provides systemic benefits by enhancing cardiac output and skeletal muscle blood flow, reducing fall risk, and modulating oxidative stress [12, 21, 22]. Our findings also align with Roy’s adaptation model of nursing, demonstrating that continuous exercise can foster adaptive behaviors in patients, improving their physiological and behavioral responses to diabetes-related stimuli [23, 24].
This study had limitations in controlling other physical activities performed by patients at home, which may have influenced the results. Additionally, due to incomplete or outdated HbA1c and lipid profile data, these variables were excluded, despite being highly relevant to diabetes complications. Patients with T2DM are recommended to regularly perform HBFAE as part of efforts to prevent diabetic foot complications. This exercise program is easy to do at home and does not require direct supervision, thus promoting patients’ independence in self-care. Healthcare facilities and professionals need to provide clear and structured education on the steps of performing HBFAEs and their benefits for foot circulation and overall health. Diabetic patients are encouraged to involve their families in supporting the implementation of this exercise program at home to ensure higher consistency and adherence.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Universitas Indonesia, Depok, Indonesia (Code: Ket-41/UN2.F12. D1.2.1/PPM.00.02/2022) and the Ethics Committee of Universitas Udayana, Sanglah General Hospital, Bali, Indonesia (Code: 381/UN.14.2.2.VII.14/LT/2022), which was accredited by the International Convention on Harmonization of Good Clinical Trial Practice (ICH-GCP) on 24 February 2022. It also has a registered clinical trial ID (Code: INA-3TBDPZ1L; DATED: 01-04-2023). Respondents were assured of their rights and privacy and provided compensation in the form of access to a medication fund in the event of injury. Before conducting the study, all participants declared their written informed consent.
Funding
This study was extracted from a master’s thesis of I Putu Adi Suryawan, approved by Universitas Indonesia, and the Sanglah General Hospital (No.: LB.02.01/XIV.2.2.1/101904/2022), funded by the Publication International Indexed 2022 (PUTI 2022) (Grant No.: NKB-087/UN2.RST/HKP.05.00/2022).
Authors' contributions
Study design: Debie Dahlia; Investigation and writing the original draft: I Putu Adi Suryawan; Supervision, review and editing: Dikha Ayu Kurnia and Debie Dahlia; Statistical analysis: I Putu Adi Suryawan and Debie Dahlia; Final approval: All author.
Conflict of interest
The authors declared no conflict of interest
Acknowledgments
The authors would like to thank the Faculty of Nursing, Universitas Indonesia, the Endocrine Polyclinic of Sanglah General Hospital in Denpasar, and all the patients who participated in this study.
References
uncontrolled blood glucose is a major risk factor for complications in patients with Diabetes Mellitus (DM), including those with Peripheral Arterial Disease (PAD) and Diabetic Foot Ulcers (DFUs). Physical exercise is a key non-pharmacological therapy to improve glycemic control and peripheral circulation [1, 2]. During the COVID-19 pandemic, reduced physical activity among DM patients further heightened the risk of foot complications, drawing attention to home-based interventions [3, 4]. Globally, the prevalence of DM is rising rapidly, projected to reach 21.3 million by 2030 [5]. In Indonesia, the national DM prevalence increased from 6.9% in 2013 to 8.5% in 2018, with Denpasar in Bali reporting the highest rate (2%) [6]. PAD affected approximately 236 million individuals aged ≥40 years worldwide in 2019, predominantly in low-income countries [7]. In Indonesia, the incidence rate is 13,807 per one million population [6]. PAD in DM patients, if unaddressed, leads to reduced Ankle Brachial Index (ABI) values and a higher risk of DFUs [8, 9].
Standard diabetic foot exercises, commonly promoted in health services, focus primarily on ankle movements and lack components needed to improve circulation throughout the foot. In contrast, Home-Based Foot-Ankle Exercise (HBFAE) targets all foot and ankle muscles [10, 11]. The difference between the HBFAE and the standard diabetic foot exercise is that the latter consists of only two exercise categories, namely stretching and strengthening exercises, which are further divided into five types of exercise, whereas the former consists of four exercise categories, namely stretching, strengthening, resistance, and balance exercises, which are further divided into ten types of exercise. This is in line with Colberg et al.’s recommendations, emphasizing the combination of aerobic, resistance, and flexibility/balance exercises for optimal outcomes [12]. A preliminary study revealed that, although the patients regularly performed standard foot exercises, many continued to experience foot complaints such as numbness and tingling. Respondents expressed the need for more effective exercise methods that could strengthen the calf, foot, and ankle muscles [13, 14]. A study suggested that resistance training may positively affect both vascular and neurological outcomes in DM patients [15]. Resistance training can significantly improve muscle strength, dynamic balance, and physical function in DM patients with comorbid knee osteoarthritis [16].
The results regarding the HBFAE effects are contradictory. For example, Silva et al. reported that an 8-week HBFAE did not significantly reduce modifiable DFU risk factors, though it helped maintain gait stability over 16 weeks [13]. Given these discrepancies, further investigation is needed to assess the potential benefits of HBFAE, particularly regarding its impact on the ABI value. Therefore, this study aims to evaluate the effectiveness of HBFAE in improving ABI values of patients with Type 2 DM (T2DM).
Materials and Methods
This is a randomized clinical trial that follows the CONSORT 2010 guidelines [17]. Participants were 40 patients with T2DM who visited the Diabetes Polyclinic in Denpasar and Badung Regency, Indonesia, for diabetes control. They were included in the study after signing an informed consent form and meeting the following inclusion criteria: Having an ABI value <0.9 in one or both extremities, experiencing one or more peripheral perfusion complaints in the foot (such as tingling, numbness, burning sensation, and feeling as if wearing socks), ability to walk without assistance for at least 10 m, age 40-65 years, having at most one amputated toe (other than the hallux), residency in Denpasar and Badung Regency, Indonesia (study location), and having at least one family member willing to supervise the exercise intervention. The exclusion criteria were having an active plantar ulcer (diabetic foot) and a history of surgery to the knee, ankle, or hip. Participants were then randomly assigned to equal groups: 20 in the intervention group and 20 in the control group. Randomization was carried out by an individual independent of the research team using the block randomization method. Each block contained four subjects, resulting in six variations of blocks. Both the researchers and participants were unaware of the group allocation. The computer-generated random numbers were placed in an opaque envelope and assigned based on the respondent’s arrival sequence number. Two participants left the study. Finally, 38 participants were assessed: 19 in the intervention group and 19 in the control group (Figure 1). To estimate the sample size, we used the following parameters adapted from a similar study conducted by Barone Gibbs et al. [18]. The standard deviation of the intervention and control groups was considered 0.14 and 0.02, respectively. The combined standard deviation of both groups after calculation was 0.095, and the mean difference between the intervention and control groups was 0.115. Type I error was set at 5% (two-tailed hypothesis), resulting in Zα=1.96, and Type II error was set at 10%, resulting in Zβ=1.645, considering a test power of 80%. The sample size was then estimated to be 36 participants, and 10% was added to account for potential dropouts, resulting in an estimated sample size of 40.
Three instruments were used in this research: a questionnaire surveying the respondent’s characteristics, including gender, smoking history, and hypertension history, a calibrated glucometer for blood glucose measurement, and a calibrated sphygmomanometer and 8-MHz portable Doppler probes for ABI measurement. The sphygmomanometer was calibrated by comparing its measurements with those of a verified standard mercury sphygmomanometer, performed by trained personnel. The 8 MHz portable Doppler was calibrated by checking the sound quality and accuracy of pressure readings, ensuring that the device could clearly detect blood flow. Recalibration was performed weekly during the data collection period to maintain measurement consistency. The assessors, who were not involved in delivering the intervention, conducted the pre-test and post-test ABI assessments. The assessors were blinded to the group allocation. They were previously trained on how to measure ABI, and their inter-rater agreement was assessed using the kappa test (kappa value=0.874). The ABI measurement was taken one day before the intervention (pre-test) and one day after the 24th intervention (post-test). Researchers checked blood sugar twice a week before exercise and when there were signs & symptoms of hypoglycemia in patients (they had previous education about the signs and symptoms of hypoglycemia, and its prevention and treatment methods). For those with blood glucose levels <100 mg/dL, training was delayed, and hypoglycemic therapy was administered first.
The intervention group was asked to perform the HBFAEs, including four exercises divided into ten types. These exercises included foot/ankle stretching and ankle range-of-motion exercises, foot/ankle strengthening, foot/ankle resistance training, and balance exercises. The HBFEAs were performed once a day, 10 movements of 3-5 minutes each day (total time=30-50 minutes per exercise). The control group performed the standard diabetic foot exercise, which consisted of seven types of exercises. Both groups underwent treatment five times a week for 24 sessions (5 weeks). The researchers monitored the implementation of the HBFEAs and diabetic foot exercises by visiting the respondents’ houses twice a week, or through videocalling a family member, three times a week. Two co-researchers who already had a good clinical practice certificate assisted the researchers. Research assistants assisted the main researcher in training, supervising, and conducting home visits to observe the implementation of the HBFEA and diabetic foot exercises. The level of agreement between the researchers and assistants regarding the implementation of the HBFEA and diabetic foot exercises was tested using the Kappa test. The kappa coefficient value obtained for the agreement between the main researcher and assistant 1 was 0.794 (>0.6), and the kappa coefficient value between the main researcher and assistant 2 was 0.659 (>0.6).
The statistical analysis was performed in SPSS software, version 23. frequency and Mean±SD were used to describe the data. Furthermore, differences in the ABI values after treatment between the control and intervention groups were determined using an independent t-test. Differences in ABI values within groups were analyzed using a paired t-test. P<0.05 was considered statistically significant. The data for the normal distribution were tested using the Shapiro-Wilk test. The N-gain score was calculated to assess the effectiveness of therapy based on the ideal ABI value. The multivariate regression analysis was used to find the variables that can predict the changes in ABI values.
Results
During the research process, none of the respondents experienced injuries or complications from the exercise intervention. The demographic/clinical characteristics of the participants, including gender, smoking history, history of foot ulcers, and history of hypertension, are shown in Table 1.

The majority of respondents in both groups were male (57.9% in the intervention group and 68.4% in the control group), with no smoking history (73.7% in the intervention group and 57.9% in the control group), with no history of hypertension (68.4% in the intervention group and 57.9% in the control group), and with no history of foot ulcers (84.2% in the intervention group and 89.5% in the control group). The results of the chi-square test showed no significant differences between the two groups in terms of gender, smoking history, hypertension history, and history of foot ulcers. Other respondent characteristics, including random blood glucose levels and DM disease duration, are presented in Table 2.

The mean blood glucose level was 147±23.21 mg/dL in the intervention group and 168±33.17 mg/dL in the control group; and had been diagnosed with diabetes for 6 years (intervention and control group).
The mean ABI values for the intervention and control groups are presented in Table 3.

The results of the independent t-test indicated that both therapies increased the ABI value significantly (P<0.05), where the difference in mean ABI value in the intervention group was greater than that in the control group (0.15 vs 0.03). In the N-gain score test, the ideal ABI value was determined to be 1.1. According to the N-gain score test results, the HBFAE had an effectiveness score (effect size) of 0.72 (72%), which suggests that the therapy was highly effective (g>0.7), whereas the diabetic foot exercise had an effectiveness score (effect size) of 0.14, indicating that the standard diabetic foot exercise was not effective (g<0.3). The mean difference in ABI value between the intervention and control groups after intervention was significant (Mean difference=0.12, 95% CI; 0.08%, 0.17%, P=0.001), as shown in Table 4.

The results of multivariate regression analysis are presented in Table 5.

In the final regression model, four confounding variables with a difference >10% in OR during the variable selection process were included. The results showed these four variables (random blood glucose level, DM duration, foot ulcer history, and smoking history) did not have any significant association with the ABI value (P>0.05).
Discussion
This study found that the HBFAE was more effective than the standard diabetic foot exercise in increasing the ABI value. The greater effectiveness of HBFAE can be attributed to its comprehensive structure, which includes stretching, strengthening, resistance, and balance exercises. These components target all foot and ankle muscles, enhancing both local and systemic vascularization [13, 19]. Several studies have also reported the benefits of resistance and balance exercises in improving muscle strength, foot function, and circulation. According to Chen et al. resistance training with TheraBand could increase muscle strength, dynamic balance, and physical function, but its effect on the ABI value was not evaluated [16]. Gholami et al. demonstrated that a resistance-based program significantly improved the ABI values [15]. Similarly, Silva et al. reported improvements in proprioception, peripheral nerve sensitivity, and gait through balance exercises. However, although an 8-week HBFAE maintained gait stability, it did not significantly reduce modifiable risk factors for DFU [13]. Li et al. found that combining the ABI with the percentage of mean arterial pressure at the ankle significantly improved the prediction of all-cause mortality in patients with T2DM compared to using the ABI alone [20].
Resistance training promotes neurovascular recovery by increasing Schwann cell proliferation and neurotrophin expression, while balance exercises improve foot structure, motor neuropathy, and venous return. These combined effects may explain the enhanced ABI scores after HBFAE in our study. Furthermore, the HBFAE provides systemic benefits by enhancing cardiac output and skeletal muscle blood flow, reducing fall risk, and modulating oxidative stress [12, 21, 22]. Our findings also align with Roy’s adaptation model of nursing, demonstrating that continuous exercise can foster adaptive behaviors in patients, improving their physiological and behavioral responses to diabetes-related stimuli [23, 24].
This study had limitations in controlling other physical activities performed by patients at home, which may have influenced the results. Additionally, due to incomplete or outdated HbA1c and lipid profile data, these variables were excluded, despite being highly relevant to diabetes complications. Patients with T2DM are recommended to regularly perform HBFAE as part of efforts to prevent diabetic foot complications. This exercise program is easy to do at home and does not require direct supervision, thus promoting patients’ independence in self-care. Healthcare facilities and professionals need to provide clear and structured education on the steps of performing HBFAEs and their benefits for foot circulation and overall health. Diabetic patients are encouraged to involve their families in supporting the implementation of this exercise program at home to ensure higher consistency and adherence.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Universitas Indonesia, Depok, Indonesia (Code: Ket-41/UN2.F12. D1.2.1/PPM.00.02/2022) and the Ethics Committee of Universitas Udayana, Sanglah General Hospital, Bali, Indonesia (Code: 381/UN.14.2.2.VII.14/LT/2022), which was accredited by the International Convention on Harmonization of Good Clinical Trial Practice (ICH-GCP) on 24 February 2022. It also has a registered clinical trial ID (Code: INA-3TBDPZ1L; DATED: 01-04-2023). Respondents were assured of their rights and privacy and provided compensation in the form of access to a medication fund in the event of injury. Before conducting the study, all participants declared their written informed consent.
Funding
This study was extracted from a master’s thesis of I Putu Adi Suryawan, approved by Universitas Indonesia, and the Sanglah General Hospital (No.: LB.02.01/XIV.2.2.1/101904/2022), funded by the Publication International Indexed 2022 (PUTI 2022) (Grant No.: NKB-087/UN2.RST/HKP.05.00/2022).
Authors' contributions
Study design: Debie Dahlia; Investigation and writing the original draft: I Putu Adi Suryawan; Supervision, review and editing: Dikha Ayu Kurnia and Debie Dahlia; Statistical analysis: I Putu Adi Suryawan and Debie Dahlia; Final approval: All author.
Conflict of interest
The authors declared no conflict of interest
Acknowledgments
The authors would like to thank the Faculty of Nursing, Universitas Indonesia, the Endocrine Polyclinic of Sanglah General Hospital in Denpasar, and all the patients who participated in this study.
References
- Kurnia DA, Dahlia D. [Efektifitas lembar pemantauan insulin terintegrasi untuk mengurangi kejadian hipoglikemia pada pasien diabetes mellitus tipe 2 (Indonesian)]. J Keperawatan Indones. 2018; 21:69-76. [DOI:10.7454/jki.v21i2.497]
- Yan J, Dai X, Feng J, Yuan X, Li J, Yang L, et al. Effect of 12-month resistance training on changes in abdominal adipose tissue and metabolic variables in patients with prediabetes: A randomized controlled trial. J Diabetes Res. 2019; 2019:8469739. [DOI:10.1155/2019/8469739] [PMID]
- Otsuka S, Morisawa T, Hojo Y, Ishida A, Tamaki A. Effect of home-based exercise therapy for peripheral arterial disease patients underwent endovascular treatment: A clinical controlled design. Phys Ther Res. 2021; 24(2):120-7. [DOI:10.1298/ptr.E10056] [PMID]
- Silva ÉQ, Santos DP, Beteli RI, Monteiro RL, Ferreira JSSP, Cruvinel-Junior RH, et al. Feasibility of a home-based foot-ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: Randomised controlled FOotCAre (FOCA) Trial II. Sci Rep. 2021; 11(1):12404. [DOI:10.1038/s41598-021-91901-0] [PMID]
- van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: One year results from the first international, open-label, prospective, multicentre study. Gut. 2020; 69(2):295-303. [DOI:10.1136/gutjnl-2019-318349] [PMID]
- Kementrian Kesehatan RI. [Hasil utama riset kesehatan dasar 2018 (Indonesian)]. Jakarta: Kementrian Kesehatan RI Badan Penelitian dan Pengembangan Kesehatan; 2018. [Link]
- Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob Health. 2019; 7(8):e1020-30. [DOI:10.1016/S2214-109X(19)30255-4] [PMID]
- Abbade LPF, Frade MAC, Pegas JRP, Dadalti-Granja P, Garcia LC, Bueno Filho R, et al. Consensus on the diagnosis and management of chronic leg ulcers - Brazilian society of dermatology. An Bras Dermatol. 2020; 95(Suppl 1):1-18. [DOI:10.1016/j.abd.2020.06.002] [PMID]
- Najafi B, Reeves ND, Armstrong DG. Leveraging smart technologies to improve the management of diabetic foot ulcers and extend ulcer-free days in remission. Diabetes Metab Res Rev. 2020 ; 36(Suppl 1):e3239. [DOI:10.1002/dmrr.3239] [PMID]
- Lai YC, Chao YH, Kuo CY, Lee WN, Chuang L, Shih TTF,et al. Microcirculatory responses to muscle and tendon exercises in individuals with and without type 2 diabetes mellitus and the association between microcirculatory and exercise performance. Metab Syndr Relat Disord. 2021; 19(6):325-31. [DOI:10.1089/met.2020.0111] [PMID]
- Minnock D, Annibalini G, Le Roux CW, Contarelli S, Krause M, Saltarelli R, et al. Effects of acute aerobic, resistance and combined exercises on 24-h glucose variability and skeletal muscle signalling responses in type 1 diabetics. Eur J Appl Physiol. 2020; 120(12):2677-91. [DOI:10.1007/s00421-020-04491-6] [PMID]
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2016; 39(11):2065-79. [DOI:10.2337/dc16-1728] [PMID]
- Silva ÉQ, Veríssimo JL, Ferreira JSSP, Cruvinel-Júnior RH, Monteiro RL, Suda EY, et al. Effects of a home-based foot-ankle exercise program with educational booklet for foot dysfunctions in people with diabetic neuropathy: Results of the FOCA-II randomized controlled clinical trial. Appl Sci. 2023; 13(3):1423. [DOI:10.3390/app13031423]
- Suwannasrisuk P, Sattanon S, Taburee W, Singkheaw P, Sowanna N, Boonprasert P, et al. Prevalence and predictors of peripheral arterial disease determined by ankle brachial index in diabetes population treated within primary care services in a non-urban area of lower northern Thailand. Diab Vasc Dis Res. 2020; 17(6):1479164120966997. [DOI:10.1177/1479164120966997] [PMID]
- Gholami F, Khaki R, Mirzaei B, Howatson G. Resistance training improves nerve conduction and arterial stiffness in older adults with diabetic distal symmetrical polyneuropathy: A randomized controlled trial. Exp Gerontol. 2021; 153:111481. [DOI:10.1016/j.exger.2021.111481] [PMID]
- Chen SM, Shen FC, Chen JF, Chang WD, Chang NJ. Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. Int J Environ Res Public Health. 2019; 17(1):224. [DOI:10.3390/ijerph17010224] [PMID]
- Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019; 13(Suppl 1):S27-30. [DOI:10.4103/sja.SJA_559_18] [PMID]
- Barone Gibbs B, Dobrosielski DA, Althouse AD, Stewart KJ. The effect of exercise training on ankle-brachial index in type 2 diabetes. Atherosclerosis. 2013; 230(1):125-30. [DOI:10.1016/j.atherosclerosis.2013.07.002] [PMID]
- Reusch JE, Regensteiner JG, Stewart KJ, Veves A. Diabetes and exercise from pathophysiology to clinical implementation. London: Springer Nature; 2018. [DOI:10.1007/978-3-319-61013-9]
- Li YH, Sheu WH, Lee IT. Use of the ankle-brachial index combined with the percentage of mean arterial pressure at the ankle to improve prediction of all-cause mortality in type 2 diabetes mellitus: An observational study. Cardiovasc Diabetol. 2020; 19(1):173. [DOI:10.1186/s12933-020-01149-7] [PMID]
- Morrison S, Simmons R, Colberg SR, Parson HK, Vinik AI. Supervised balance training and wii fit-based exercises lower falls risk in older adults with type 2 diabetes. J Am Med Dir Assoc. 2018; 19(2):185.e7-13. [DOI:10.1016/j.jamda.2017.11.004] [PMID]
- Koca N, Güçlü M, Esen İ, Emlek G, Kiyici S, Kisakol G. Physical activity, insulin sensitivity, and metabolic control in type 1 diabetes mellitus. Turk J Endocrinol Metab. 2019; 23:8-18. [Link]
- Abdolahi M, Doustmohamadi MM, Sheikhbardsiri H. The effect of an educational plan based on the roy adaptation model for fatigue and activities of daily living of patients with heart failure disease. Ethiop J Health Sci. 2020; 30(4):559-66. [DOI:10.4314/ejhs.v30i4.11] [PMID]
- Majeed I, Sehar S, Afzal M, Gilani SA, Parveen K, Ahmed R. Effect of Roy’s adaptation model based interventions on quality of life in patients with type II diabetes. Pure Appl Biol. 2020; 9(1):332-9. [DOI:10.19045/bspab.2020.90038]
Article Type : Research |
Subject:
General
Received: 2023/04/23 | Accepted: 2025/09/8 | Published: 2025/09/8
Received: 2023/04/23 | Accepted: 2025/09/8 | Published: 2025/09/8
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |