Sat, May 4, 2024
Volume 33, Issue 2 (3-2023)
JHNM 2023, 33(2): 159-169 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Chouhdari A, Rezaei O, Samadian M, Sharifi G, Ebrahimzadeh K, Davoudi Z. Comparing Quality of Life Before and After Surgery in Patients With Cushing Disease: A Before-After Study. JHNM 2023; 33 (2) :159-169
URL: http://hnmj.gums.ac.ir/article-1-2071-en.html
URL: http://hnmj.gums.ac.ir/article-1-2071-en.html
Arezoo Chouhdari1 

 , Omidvar Rezaei2
, Omidvar Rezaei2 

 , Mohammad Samadian2
, Mohammad Samadian2 

 , Guive Sharifi2
, Guive Sharifi2 

 , Kaveh Ebrahimzadeh3
, Kaveh Ebrahimzadeh3 

 , Zahra Davoudi *
, Zahra Davoudi * 

 4
4


 , Omidvar Rezaei2
, Omidvar Rezaei2 

 , Mohammad Samadian2
, Mohammad Samadian2 

 , Guive Sharifi2
, Guive Sharifi2 

 , Kaveh Ebrahimzadeh3
, Kaveh Ebrahimzadeh3 

 , Zahra Davoudi *
, Zahra Davoudi * 

 4
4
1- Assistant Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Social Determinants of Health Research Center (SDHRC), Amir-al-Momenin Hospital, Tehran Medical Sciences Branch, Tehran, Iran
2- Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Assistant Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4- Assistant Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran , dr.daavoudi@sbmu.ac.ir
2- Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Assistant Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4- Assistant Professor, Skull Base Research Center, Loghman Hakim Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran , dr.daavoudi@sbmu.ac.ir
Full-Text [PDF 457 kb]
(249 Downloads)
| Abstract (HTML) (560 Views)
Full-Text: (144 Views)
Introduction
Materials and Methods

.png)
There was a significant association between the improvement score of QoL in the Tuebingen CD-25 questionnaire (Table 3)

Discussion
There are numerous general questionnaires to determine improvement in HRQOL in patients with Cushing disease, but the Tubingen CD-25 questionnaire is specific for the CD. In the present study, the Persian Tuebingen CD-25 questionnaire was used for the first time to investigate HRQOL before and after the surgery for Cushing disease. After a successful treatment process, improvement may take months, even more than a year.
This survey revealed an improved score of QoL in all dimensions of the Tuebingen CD-25 questionnaire during one-year post-surgery, but there was no difference between remission and non-remission groups. Perhaps, because of the economic factors and lack of social support that was common to each group. Milian reported that the time since surgery did not affect postoperative QoL before a few months had elapsed since the operation [16]. Some other studies did not find a correlation between QoL and months passed since surgery in the CD [26, 27]. The strength of this study was that all patients were evaluated for QoL before surgery and then 6 and 12 months after surgery. A limitation of this survey is related to the single-center data setting. Because this research was conducted only in one teaching hospital, patients may have had a lower socioeconomic status that could effectively improve the QoL scores. It will be very useful if the pituitary adenoma data are collected throughout the country and recorded in a single system that all physicians and researchers can use.
Based on these findings, many factors besides the surgery can affect the quality of life in patients with Cushing disease. Therefore, more studies are needed on various aspects of improving the quality of life of patients after surgery and other treatments in the patients.
This study was financially supported by Skull Base Research Center, Loghman Hakim Medical Center, and Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
References
Cushing Disease (CD) is known as hypercortisolism, caused by an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma [1]. The CD prevalence was 2.2 per 100000, while the incidence rate was 0.24 per 100000 person-years. Endogenous glucocorticoid excess causes different symptoms and signs in the patient, including weight gain, central obesity, buffalo hump, moon face, striae, thinning and fragile skin, acne, hirsutism or amenorrhea in women and decreased libido, fertility, and erectile dysfunction in men [2, 3]. These symptoms can negatively influence a patient’s well-being and quality of life due to changes in appearance and mental health [3, 4]. Transsphenoidal selective adenectomy is the best primary therapy for pituitary-dependent Cushing disease [4]. Physicians expect that with normalizing hormonal levels after sufficient treatment, the patient’s physical and mental health will improve, and they will return to normal daily activities [5]. However, even after an endocrine cure, patients’ scores on general well-being, anxiety, depression, and overall quality of life (QoL) are still low [5, 6]. Andela indicated that structural and functional brain abnormalities were shown to be persistent after the biochemical cure of Cushing disease [6]. Also, Asadi-Lari, in one survey, showed that CD patients had a lower QoL score than other patients with pituitary adenoma [7]. Multiple regression analysis in another study demonstrated that remission status, time to diagnosis, radiation therapy, and hypopituitarism were significant predictors of QoL improvement [8]. Also, there was no association between QoL and the patient’s sex, age, replacement steroid use, follow-up with an endocrinologist, or surgical approach. Although returning to normal life after surgery and medication is very important for patients with Cushing disease [8, 9], few studies have been performed on the epidemiology of the disease and the quality of life of patients in Iran [7, 10]. We compared QoL scores before, 6, and 12 months after surgery in the CD patients and determined predictor factors for QoL score improvement in patients.
Materials and Methods
This before-after study, due to the low prevalence of the disease, was conducted on all 56 patients 18 years and older with the Cushing disease diagnosis who underwent trans-sphenoidal surgery during the 3 years from 2017 to 2020 at one educational and medical center of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Patient’s information, including demographic characteristics (age, sex, marital status, educational level, body mass index (BMI), waist-hip ratio (WHR), tumor size, duration between symptom onset and diagnosis, re-surgery, radiotherapy, lifestyle, and past medical history (smoking, diabetes mellitus [DM]), hypertension (HTN), fracture history, signs, and symptoms were collected in a data collection form prepared by researchers. Also, hormone profiles consisting of cortisol, ACTH, urine-free cortisol (UFC) before and after surgery, and overnight dexamethasone suppression test (ODST) after surgery and other pituitary axes function (gonad, thyroid, growth axes) for monitoring remission were assessed. Biochemical remission was determined based on the 2021 guidelines for Cushing syndrome [11 12]. The remission phase is characterized by morning serum cortisol value <5 μg/dL or UFC<20 μg/dL within 7 days post-surgery and ODST cortisol level <50 nmol/L equivalent to 1.8 μg/dL.
A specific questionnaire (Tuebingen CD-25) was used to evaluate the effect of the disease and its treatment on the patient’s QoL. The Tuebingen CD-25 consists of 25 sections that cover 6 essential domains of health-related (HR) QoL: depression (6 sub-dimensions), sexual activity (4 sub-dimensions), environment (6 sub-dimensions), eating behavior (4 sub-dimensions), bodily restriction (3 sub-dimensions), and cognition (2 sub-dimensions). The total score has a minimum score of 0 and a maximum score of 100. The answers to each question were scored on the Likert scale. Also, a higher score means a lower quality of life score.The Tuebingen CD-25 questionnaire was first used in 2012 in a European study. The reliability and validity of the Tuebingen CD-25 questionnaire have been confirmed in English [13].
To customize the Tuebingen CD-25 questionnaire in assessing the quality of life of patients with Cushing disease, first, the English version of this questionnaire was translated into Persian by four translators using the forward-backward method, and then its face and content validities were assessed. To evaluate the content validity, the Lawshe model was used. Following this method, the questionnaire was sent to 9 specialists, including neurosurgeons, psychologists, and social medicine, to ask their opinions in three areas of necessity, clarity, and simplicity of questions. Then, the panel members’ votes were quantified by calculating the content validity ratio (CVR), and by placing in the content validity index (CVI) calculation formula, the final index number 0.99 was obtained. To determine the face validity, the final version of the questionnaire, after confirming the content validity, was given to 15 patients, and they were asked about the relevance, simplicity, and clarity of the questions on a 4-point scale format. The 4 points were as follows: 1) completely relevant, simple, and clear; 2) relevant, simple, and clear but requires minor revisions; 3) requires major revision; and 4) completely irrelevant. Each question was confirmed if 50% of the patients chose either the first option or 70% chose the first two options. Therefore, face validity was also confirmed [14, 15]. The Guttman split-half coefficient was used to evaluate the reliability and correlation coefficient: estimated r=0.9.
The Tuebingen CD-25 questionnaire was completed by patients first before the surgery in the pituitary clinic and then 6 and 12 months after surgery at the time of face-to-face follow-up. If the patients could not attend in person, the questionnaire was completed by researchers via phone. Surgery was performed by a skilled neurosurgeon with at least 5 years of surgical experience. All patients completed the informed consent form at the beginning of the study. If the patient were not available after surgery for any reason, he would be excluded from the study. The patients were informed and explained about the confidentiality of their information. In this study, to report basic descriptive variables of demographics, signs, and symptoms, as well as each dimension of the Tuebingen CD-25 questionnaire, number, percentage, and the mean ± standard deviation (SD) were applied. For comparison of the Tuebingen CD-25 questionnaire score between three time points (before, 6, and 12 months after surgery), repeated measures ANOVA was used. To evaluate associated factors with QoL score improvement, the independent t test and bivariate Pearson correlation test (correlation coefficient indicated by r) were used. Also, to predict possible factors for more improvement of QoL score in CD patients after surgery, the median improvement score was first estimated and then divided into two groups, and multivariable logistic regression analysis was performed. Analyzes were performed (odds ratio, 95% confidence interval) in SPSS version 19 software. The statistical significance level P<0.05 was considered.
Results
A specific questionnaire (Tuebingen CD-25) was used to evaluate the effect of the disease and its treatment on the patient’s QoL. The Tuebingen CD-25 consists of 25 sections that cover 6 essential domains of health-related (HR) QoL: depression (6 sub-dimensions), sexual activity (4 sub-dimensions), environment (6 sub-dimensions), eating behavior (4 sub-dimensions), bodily restriction (3 sub-dimensions), and cognition (2 sub-dimensions). The total score has a minimum score of 0 and a maximum score of 100. The answers to each question were scored on the Likert scale. Also, a higher score means a lower quality of life score.The Tuebingen CD-25 questionnaire was first used in 2012 in a European study. The reliability and validity of the Tuebingen CD-25 questionnaire have been confirmed in English [13].
To customize the Tuebingen CD-25 questionnaire in assessing the quality of life of patients with Cushing disease, first, the English version of this questionnaire was translated into Persian by four translators using the forward-backward method, and then its face and content validities were assessed. To evaluate the content validity, the Lawshe model was used. Following this method, the questionnaire was sent to 9 specialists, including neurosurgeons, psychologists, and social medicine, to ask their opinions in three areas of necessity, clarity, and simplicity of questions. Then, the panel members’ votes were quantified by calculating the content validity ratio (CVR), and by placing in the content validity index (CVI) calculation formula, the final index number 0.99 was obtained. To determine the face validity, the final version of the questionnaire, after confirming the content validity, was given to 15 patients, and they were asked about the relevance, simplicity, and clarity of the questions on a 4-point scale format. The 4 points were as follows: 1) completely relevant, simple, and clear; 2) relevant, simple, and clear but requires minor revisions; 3) requires major revision; and 4) completely irrelevant. Each question was confirmed if 50% of the patients chose either the first option or 70% chose the first two options. Therefore, face validity was also confirmed [14, 15]. The Guttman split-half coefficient was used to evaluate the reliability and correlation coefficient: estimated r=0.9.
The Tuebingen CD-25 questionnaire was completed by patients first before the surgery in the pituitary clinic and then 6 and 12 months after surgery at the time of face-to-face follow-up. If the patients could not attend in person, the questionnaire was completed by researchers via phone. Surgery was performed by a skilled neurosurgeon with at least 5 years of surgical experience. All patients completed the informed consent form at the beginning of the study. If the patient were not available after surgery for any reason, he would be excluded from the study. The patients were informed and explained about the confidentiality of their information. In this study, to report basic descriptive variables of demographics, signs, and symptoms, as well as each dimension of the Tuebingen CD-25 questionnaire, number, percentage, and the mean ± standard deviation (SD) were applied. For comparison of the Tuebingen CD-25 questionnaire score between three time points (before, 6, and 12 months after surgery), repeated measures ANOVA was used. To evaluate associated factors with QoL score improvement, the independent t test and bivariate Pearson correlation test (correlation coefficient indicated by r) were used. Also, to predict possible factors for more improvement of QoL score in CD patients after surgery, the median improvement score was first estimated and then divided into two groups, and multivariable logistic regression analysis was performed. Analyzes were performed (odds ratio, 95% confidence interval) in SPSS version 19 software. The statistical significance level P<0.05 was considered.
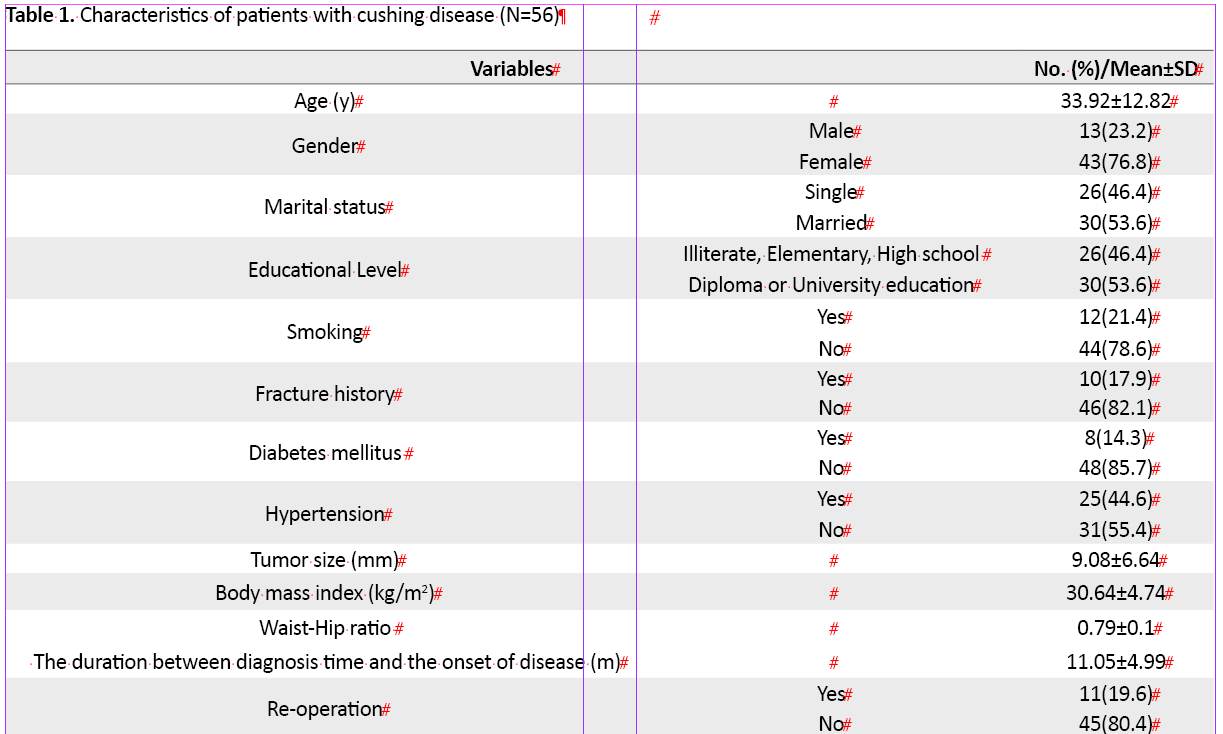
Of 56 patients with Cushing disease who underwent transsphenoidal surgery, 43 (76.8%) were female. The mean±SD age of the samples was 33.92±12.82 years (min:14, max: 55 years). Also, 30 patients (53.6%) were married, and the educational level of 30 patients (53.6%) was diploma and higher. Their mean ±SD tumor size was 9.08±6.64 mm (min: 1 and max: 25 mm). Eleven patients (19.6%) underwent re-operation. Conventional radiotherapy was performed in 5 patients (8.9%) after surgery. Total characteristics before and after surgery are presented in Table 1.



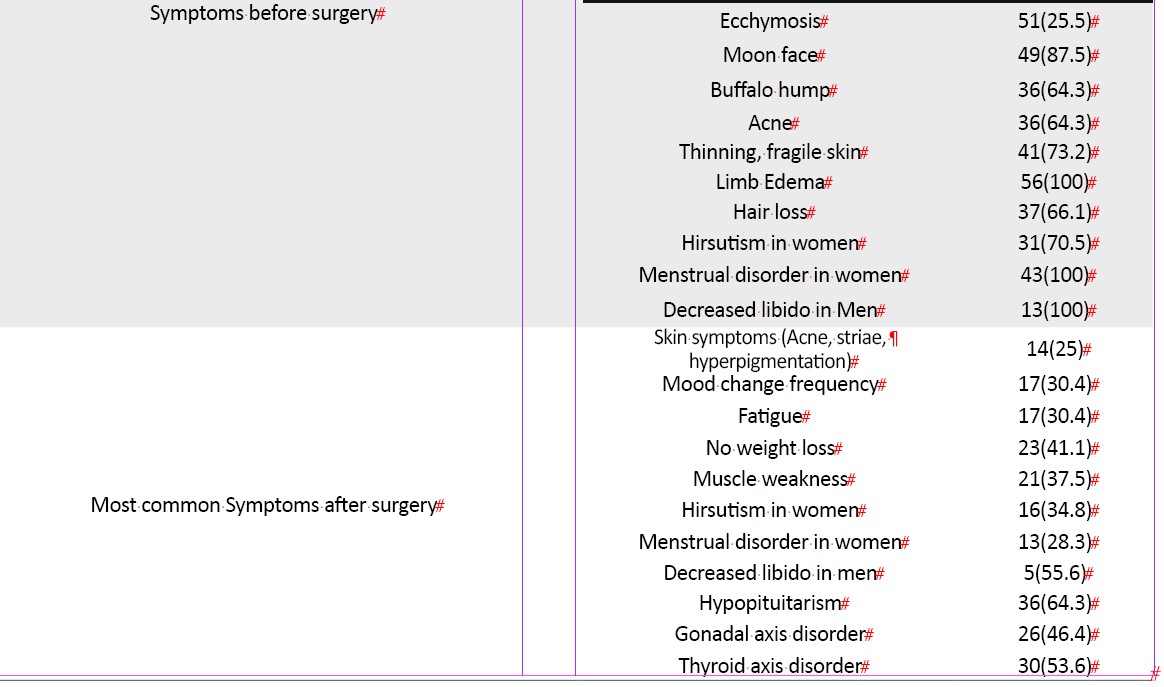
The levels of UFC [(before: 839.65±630.5, after: 44.96±12.88 mg/dl), ACTH (before: 94.03±86.57, after: 27.24±36.73 mg/dl), ODST (only assessed after surgery: 1.90±2.6 mg/dl)] decreased dramatically after first or second surgery during one year. Most patients (71.4%) were in the remission phase for one year after surgery. Overall, the mean and median of the QoL improvement score during one year were 14.38±6.44 and 13, respectively. The mean ±SD QoL improvement score in the remission phase was 15.88±6.35, while in the patients who were not in the remission phase during one year, it was 13.77±6.52. Postoperative scores of QoL in the Tuebingen CD-25 questionnaire significantly improved in all dimensions (Table 2).
.png)
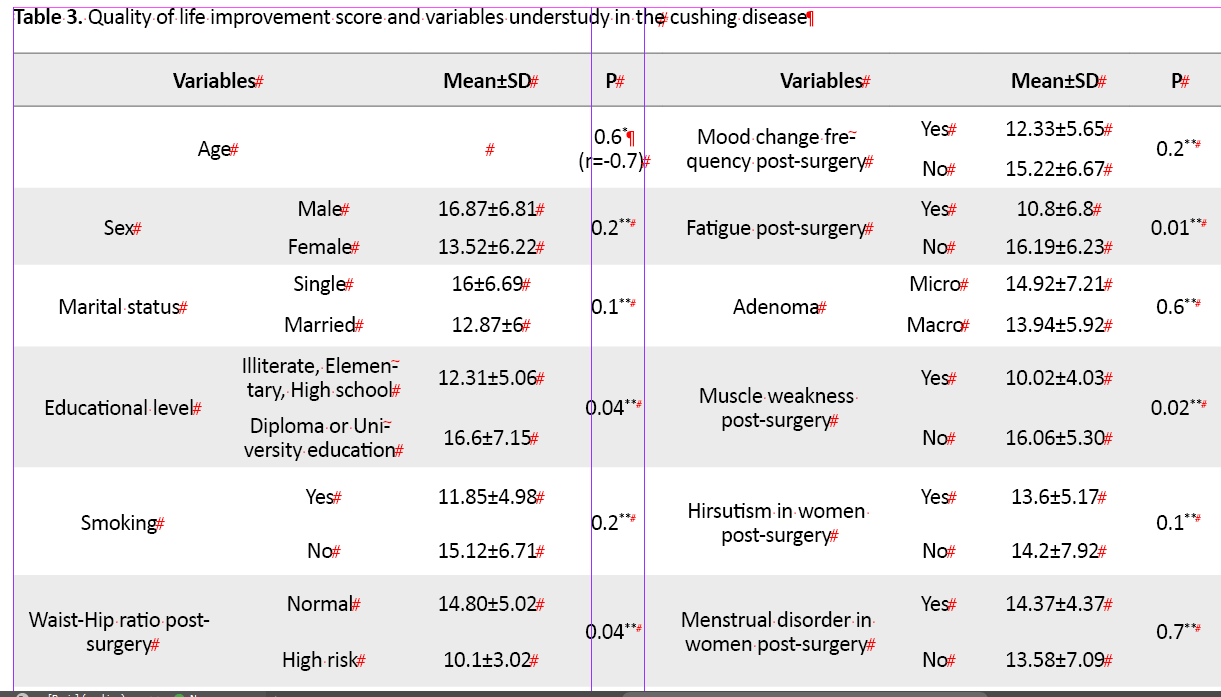
There was a significant association between the improvement score of QoL in the Tuebingen CD-25 questionnaire (Table 3)


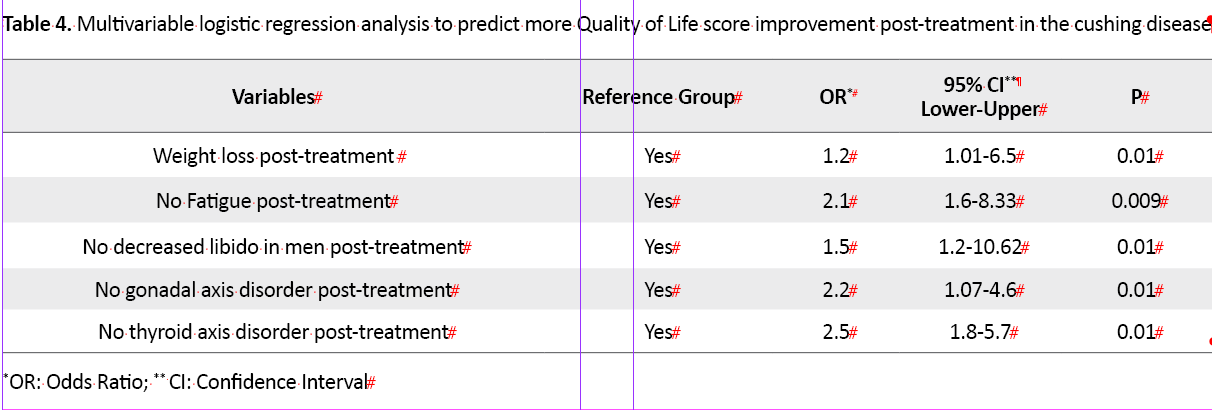
and educational level (P=0.04), high-risk WHR post-surgery (P=0.04), no weightless after surgery (P=0.002), fatigue (P=0.01), muscle weakness and inability to do daily activities after surgery (P=0.02), and decreased libido in men (P=0.02). In multivariable logistic regression analysis, weight loss post-treatment 1.2 times (OR=1.2, 95% CI; 1.01-6.5, P=0.01), no fatigue post-treatment 2.1 times (OR= 2.1, 95% CI; 1.6-8.3, P=0.009,), no decreased libido 1.5 times (OR= 1.5, 95%CI; 1.2-10.62, P=0.01), no gonadal axis disorder 2.2 times (OR=2.2, 95% CI;1.07-4.06, P=0.01) and no thyroid axis disorder 2.2 times (OR=2.5, 95% CI;1.8-5.7, P=0.01) post-treatment were predictor factors for more QoL score improvement during one year after surgery (Table 4)

Discussion
There are numerous general questionnaires to determine improvement in HRQOL in patients with Cushing disease, but the Tubingen CD-25 questionnaire is specific for the CD. In the present study, the Persian Tuebingen CD-25 questionnaire was used for the first time to investigate HRQOL before and after the surgery for Cushing disease. After a successful treatment process, improvement may take months, even more than a year.
We found significant improvement in QoL scores in all domains of Tubingen CD-25 questionnaires, but despite the significant reduction in hormone levels, the difference between domains’ scores during one year was lower than Milian et al. survey [16]. Many factors, such as economic conditions and social support, can affect the quality-of-life score after treatment. We found the lowest QoL score improvement in sexual activity and cognition behavior dimensions. It can be explained by the fact that the dimension of sexual activity with 4 sub-dimensions and the dimension of cognitive behavior with two sub-dimensions had the worst conditions even before the treatment of patients. In the Milian et al. study, however, the depression score was impaired even after successful hormonal treatment [16]. Previous studies indicated memory and concentration impairments in patients with unsuccessful hormonal therapy [17, 18]. They found that persistent cognitive impairment among CD patients in the remission phase was due to exposure to high levels of glucocorticoids that could have detrimental effects on cognitive behavior over time.
In this study, although patients in the remission phase in terms of pituitary axes had better QoL scores, this improvement was not significant. In contrast, some other studies showed that patients in the remission phase of CD have better QoL scores than active patients measured with disease-specific and generic questionnaires [19, 20]. Although current research pointed out no significant correlation between age and quality of life improvement score, in the Van Aken study, a decreasing score in QoL with advancing age was seen for physical activity [18]. Also, Milian et al. mentioned that younger patients between 21 and 30 years had restrictions in health-related quality of life predominantly in the dimensions of sexual activity, cognition behavior, social environment, and eating behavior, while impairments in the domain of bodily restrictions were more seen in patients between 51 and 70 years [16].
We found a significant association between QoL improvement score and educational level, which was new in the pituitary adenoma QoL research studies. Overall, more positive feelings, less dependence on medication and treatment, and better physical and social activity represent QoL advantages to those who received academic education compared with elementary school [19]. Developing countries reported poorer environmental, psychological, and physical QoL than developed countries [20]. Other studies indicated better QoL improvement after surgery at a higher level of education [21, 22]. In the Hook study, overweight women had a poorer obesity-related QoL than normal-weight women [23]. Also, Another study showed that in people with higher BMI, improvement of QoL score is rare in CD [19]. Although there was no association between QoL improvement and smoking, DM, or HTN but some post-surgery comorbidities such as fatigue, muscle weakness, decreased libido, and gonadal and thyroid axis disorders were related to lower QoL score improvement. Generally, pituitary axis dysfunction contributes to the lack of improvement in a patient’s quality of life after surgery [23, 24]. Some results have demonstrated that comorbidities can also have an important effect on HRQoL. Having more than two post-surgery comorbidities has been reported as a predictor of low QoL [25, 26]. Also, there was no difference between QoL score improvement and re-operation as well as radiotherapy in this survey. However, Santos expressed that conventional radiotherapy was one of the causes of low QoL in Cushing syndrome [19], which can be due to the need for many sessions of treatment with this method of radiotherapy. Webb study indicates that patients with depression have higher urinary cortisol levels than those without post-surgery. Also, it was reported that cured patients present with better scores in QoL than those with active disease [25].
We found a significant association between QoL improvement score and educational level, which was new in the pituitary adenoma QoL research studies. Overall, more positive feelings, less dependence on medication and treatment, and better physical and social activity represent QoL advantages to those who received academic education compared with elementary school [19]. Developing countries reported poorer environmental, psychological, and physical QoL than developed countries [20]. Other studies indicated better QoL improvement after surgery at a higher level of education [21, 22]. In the Hook study, overweight women had a poorer obesity-related QoL than normal-weight women [23]. Also, Another study showed that in people with higher BMI, improvement of QoL score is rare in CD [19]. Although there was no association between QoL improvement and smoking, DM, or HTN but some post-surgery comorbidities such as fatigue, muscle weakness, decreased libido, and gonadal and thyroid axis disorders were related to lower QoL score improvement. Generally, pituitary axis dysfunction contributes to the lack of improvement in a patient’s quality of life after surgery [23, 24]. Some results have demonstrated that comorbidities can also have an important effect on HRQoL. Having more than two post-surgery comorbidities has been reported as a predictor of low QoL [25, 26]. Also, there was no difference between QoL score improvement and re-operation as well as radiotherapy in this survey. However, Santos expressed that conventional radiotherapy was one of the causes of low QoL in Cushing syndrome [19], which can be due to the need for many sessions of treatment with this method of radiotherapy. Webb study indicates that patients with depression have higher urinary cortisol levels than those without post-surgery. Also, it was reported that cured patients present with better scores in QoL than those with active disease [25].
This survey revealed an improved score of QoL in all dimensions of the Tuebingen CD-25 questionnaire during one-year post-surgery, but there was no difference between remission and non-remission groups. Perhaps, because of the economic factors and lack of social support that was common to each group. Milian reported that the time since surgery did not affect postoperative QoL before a few months had elapsed since the operation [16]. Some other studies did not find a correlation between QoL and months passed since surgery in the CD [26, 27]. The strength of this study was that all patients were evaluated for QoL before surgery and then 6 and 12 months after surgery. A limitation of this survey is related to the single-center data setting. Because this research was conducted only in one teaching hospital, patients may have had a lower socioeconomic status that could effectively improve the QoL scores. It will be very useful if the pituitary adenoma data are collected throughout the country and recorded in a single system that all physicians and researchers can use.
Based on these findings, many factors besides the surgery can affect the quality of life in patients with Cushing disease. Therefore, more studies are needed on various aspects of improving the quality of life of patients after surgery and other treatments in the patients.
Ethical Considerations
Compliance with ethical guidelines
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethical code: IR.SBMU.RETECH.REC.1398.337).
FundingThis study was financially supported by Skull Base Research Center, Loghman Hakim Medical Center, and Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization: Arezoo Chouhdari, Zahra Davoudi, and Omidvar Rezaei, Mohammad Samadian, Guive Sharifi, and Kaveh Ebrahimzadeh; Draft preparation, resources, and investigation: Arezoo Chouhdari and Zahra Davoudi; Data analysis: Arezoo Chouhdari and Zahra Davoudi; Revision and confirmation to submit: Arezoo Chouhdari, Zahra Davoudi, Omidvar Rezaei, Mohammad Samadian, Guive Sharifi, and Kaveh Ebrahimzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Skull Base Research Center and Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for their support, cooperation, and assistance throughout the study.
References
- Pivonello R, De Leo M, Cozzolino A, Colao A. The treatment of Cushing’s disease. Endocrine reviews. 2015; 36(4):385-486. [DOI:10.1210/er.2013-1048] [PMID] [PMCID]
- Giuffrida G, Crisafulli S, Ferraù F, Fontana A, Alessi Y, Calapai F, et al. Global Cushing’s disease epidemiology: A systematic review and meta-analysis of observational studies. Journal of Endocrinological Investigation. 2022; 45(6):1235-46. [DOI:10.1007/s40618-022-01754-1] [PMID]
- Steffensen C, Bak AM, Rubeck KZ, Jørgensen JO. Epidemiology of Cushing’s syndrome. Neuroendocrinology. 2010; 92(S1):1-5. [DOI:10.1159/000314297] [PMID]
- Duarte DB, Puga F, Ribeiro I, Amaral C. Cushing’s disease and health-related quality of life: a cure for all dimensions? Endocrine Abstracts. 2022; 81:EP674. [DOI:10.1530/endoabs.81.EP674]
- Badia X, Valassi E, Roset M, Webb SM. Disease-specific quality of life evaluation and its determinants in Cushing’s syndrome: what have we learnt? Pituitary. 2014; 17(2):187-95. [DOI:10.1007/s11102-013-0484-2] [PMID] [PMCID]
- Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, et al. Mechanisms in endocrinology: Cushing’s syndrome causes irreversible effects on the human brain: A systematic review of structural and functional magnetic resonance imaging studies. European Journal of Endocrinology. 2015; 173(1):R1-14. [DOI:10.1530/EJE-14-1101] [PMID]
- Asadi-Lari M, Sadeghipour AR, Mahouzi L, Solaimani Dodaran M, Fallah A. [Assessment of the demographic characteristics and the quality of life in patients with pituitary adenoma in a referral pituitary center in Tehran in 2011 (Persian)]. Journal of Rafsanjan University of Medical Sciences. 2014; 13(8):695-704. [Link]
- Papoian V, Biller BM, Webb SM, Campbell KK, Hodin RA, Phitayakorn R. Patients’ perception on clinical outcome and quality of life after a diagnosis of Cushing syndrome. Endocrine Practice. 2016; 22(1):51-67. [DOI:10.4158/EP15855.OR] [PMID]
- Huckhagel T, Flitsch J, Rotermund R, Knospe V. Prevalence of signs and symptoms of pseudotumor Cerebri syndrome before and after transsphenoidal surgery for Cushing’s disease-a prospective consecutive case series. Experimental and Clinical Endocrinology & Diabetes. 2021; 129(6):465-72. [DOI:10.1055/a-1200-1528] [PMID]
- Malek M, Esfehanian F, Amouzegar A, Sarvghadi F, Moossavi Z, Mohajeri-Tehrani MR, et al. A survey of clinical practice patterns in diagnosis and management of Cushing’s disease in Iran. Medical journal of the Islamic Republic of Iran. 2016; 30:334. [PMCID]
- Fleseriu, M. Salivary cortisol in the diagnosis of Cushing syndrome, always more than one! Journal of the Endocrine Society. 2020; 4(10):bvaa109. [Link]
- Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Nienke R, et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. The lancet. 2021; 9(12):847-75. [DOI:10.1016/S2213-8587(21)00235-7] [PMID]
- Milian M, Teufel P, Honegger J, Gallwitz B, Schnauder G, Psaras T. The development of the Tuebingen Cushing’s disease quality of life inventory (Tuebingen CD-25). Part I: construction and psychometric properties. Clinical Endocrinology. 2012; 76(6):851-60. [DOI:10.1111/j.1365-2265.2011.04191.x] [PMID]
- Heidarnia M, Riazi-Isfahani S, Abadi A, Mohseni M. [Cross cultural adaptation and assessing validity and reliability of SERVQUAL questionnaire in hospital service quality (Persian)]. Research in Medicine. 2014; 38(2):98-105. [Link]
- Farajzadeh MA, Karimi L, Mirjavadi SA, Azimi Vahedian A, Gholami Chaharshahi S, Jafari Farjam SN, et al. Survey of the patients’ satisfaction with services provided in an Iranian Naval Hospital in 2019: A cross-sectional study. Romanian Journal of Military Medicine. 2021; 124(4):452-61. [DOI:10.21203/rs.3.rs-29151/v1]
- Milian M, Honegger J, Teufel P, Wolf A, Psaras T. Tuebingen CD-25 is a sensitive tool to investigate health-related quality of life in Cushing’s disease patients in the course of the disease. Neuroendocrinology. 2013; 98(3):188-99. [DOI:10.1159/000355622] [PMID]
- Heald A, Parr C, Gibson C, O’driscoll K, Fowler H. A cross-sectional study to investigate long-term cognitive function in people with treated pituitary Cushing’s disease. Experimental and Clinical Endocrinology & diabetes. 2006; 114(9):490-7. [DOI:10.1055/s-2006-924332] [PMID]
- Van Aken MO, Pereira AM, Biermasz R, Van Thiel SW, Hoftijzer HC, Smit JW, et al. Quality of life in patients after long-term biochemical cure of Cushing’s disease. The Journal of Clinical Endocrinology & Metabolism. 2005; 90(6):3279-86. [DOI:10.1210/jc.2004-1375] [PMID]
- Santos A, Resmini E, Martnez-Mombln MA, Crespo I, Valassi E, Roset M, et al. Psychometric performance of the CushingQoL questionnaire in conditions of real clinical practice. European journal of endocrinology. 2012; 167(3):337-42. [DOI:10.1530/EJE-12-0325] [PMID]
- Lindsay JR, Nansel T, Baid S, Gumowski J, Nieman LK. Long-term impaired quality of life in Cushing’s syndrome despite initial improvement after surgical remission. The Journal of Clinical Endocrinology & Metabolism. 2006; 91(2):447-53. [DOI:10.1210/jc.2005-1058] [PMID]
- Tiemensma J, Biermasz NR, Middelkoop HA, van der Mast RC, Romijn JA, Pereira AM. Increased prevalence of psychopathology and maladaptive personality traits after long-term cure of Cushing’s disease. The Journal of Clinical Endocrinology & Metabolism. 2010; 95(10):E129-41. [DOI:10.1210/jc.2010-0512] [PMID]
- Albarel F, Pellegrini I, Rahabi H, Baccou C, Gonin L, Rochette C, et al. Evaluation of an individualized education program in pituitary diseases: a pilot study. European Journal of Endocrinology. 2020; 183(6):551-9. [DOI:10.1530/EJE-20-0652] [PMID]
- Hook JN, Giordani B, Schteingart DE, Guire K, Giles J, Ryan K, et al. Patterns of cognitive change over time and relationship to age following successful treatment of Cushing’s disease. Journal of the International Neuropsychological Society. 2007; 13(1):21-9. [DOI:10.1017/S1355617707070051] [PMID]
- Skevington SM. Qualities of life, educational level and human development: An international investigation of health. Social Psychiatry and Psychiatric Epidemiology. 2010; 45(10):999-1009. [DOI:10.1007/s00127-009-0138-x] [PMID]
- Webb SM, Badia X, Barahona MJ, Colao A, Strasburger CJ, Tabarin A, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. European Journal of Endocrinology. 2008; 158(5):623-30. [DOI:10.1530/EJE-07-0762] [PMID]
- Chouhdari A, Rezaei O, Rezapour P, Samadian M, Shahrabi Farahani H, Hajiesmaeili M, et al. Effect of basic characteristics on improving quality of life after lumbar spine decompression surgery. Archives of Neuroscience. 2019; 6(3):e90159. [DOI:10.5812/ans.90159]
- Song HR, Park HS, Yun KE, Cho SH, Choi EY, Lee SY, et al. Gender and age differences in the impact of overweight on obesity-related quality of life among Korean adults. Obesity research & Clinical Practice. 2010; 4(1):e15-23. [DOI:10.1016/j.orcp.2009.07.003] [PMID]
Article Type : Research |
Subject:
Special
Received: 2023/01/25 | Accepted: 2023/01/30 | Published: 2023/01/30
Received: 2023/01/25 | Accepted: 2023/01/30 | Published: 2023/01/30
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



