Sat, Nov 22, 2025
Volume 35, Issue 4 (9-2025)
JHNM 2025, 35(4): 249-258 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Borjalizadeh M, Jafarzadeh-Kenarsari F, Seyednoori T, Kazemnezhad Leyli E, Zahiri Sorouri Z. Psychometric Properties of the Persian Version of the Modified Questionnaire for Quality of Care in the In-vitro Fertilization Programs From Patients’ Perspective. JHNM 2025; 35 (4) :249-258
URL: http://hnmj.gums.ac.ir/article-1-1816-en.html
URL: http://hnmj.gums.ac.ir/article-1-1816-en.html
Maryam Borjalizadeh1 

 , Fatemeh Jafarzadeh-Kenarsari *2
, Fatemeh Jafarzadeh-Kenarsari *2 

 , Tahereh Seyednoori1
, Tahereh Seyednoori1 

 , Ehsan Kazemnezhad Leyli3
, Ehsan Kazemnezhad Leyli3 

 , Ziba Zahiri Sorouri4
, Ziba Zahiri Sorouri4 




 , Fatemeh Jafarzadeh-Kenarsari *2
, Fatemeh Jafarzadeh-Kenarsari *2 

 , Tahereh Seyednoori1
, Tahereh Seyednoori1 

 , Ehsan Kazemnezhad Leyli3
, Ehsan Kazemnezhad Leyli3 

 , Ziba Zahiri Sorouri4
, Ziba Zahiri Sorouri4 


1- Midwifery (MSc), Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran.
2- Associate Professor, Department of Midwifery, Social Determinants of Health Research Center, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. ,f.kenarsari2013@gmail.com
3- Associate Professor, Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. & Associate Professor, Department of Biostatistics, School of Health, Guilan University of Medical Sciences, Rasht, Iran.
4- Professor, Department of Obstetrics & Gynecology, Reproductive Health Research Center, School of Medicine, Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Associate Professor, Department of Midwifery, Social Determinants of Health Research Center, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran. ,
3- Associate Professor, Road Trauma Research Center, Trauma Institute, Guilan University of Medical Sciences, Rasht, Iran. & Associate Professor, Department of Biostatistics, School of Health, Guilan University of Medical Sciences, Rasht, Iran.
4- Professor, Department of Obstetrics & Gynecology, Reproductive Health Research Center, School of Medicine, Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Keywords: Infertility, In Vitro Fertilization (IVF), Patient-centered care, Psychometrics, Quality of care
Full-Text [PDF 571 kb]
(159 Downloads)
| Abstract (HTML) (278 Views)
Full-Text: (72 Views)
Introduction
Quality of care refers to the degree to which the healthcare services provided to patients can enhance the desired health outcomes. To achieve quality care, healthcare services should be safe, effective, timely, efficient, equitable, integrated, needs-based, and patient-centered [1]. Focusing on patients’ values and needs (patient-centered care) is a key element in improving the quality of care [2, 3]. Patients’ perceptions are a valuable source of information for planning and evaluation of healthcare services [4]. Quality from Patients’ Perspective (QPP) can also serve as a measure of patients’ experiences with the services received and their expectations of the provided care [5].
In the field of Assisted Reproductive Technologies (ARTs), patient-centeredness is a critical dimension of high-quality care, alongside effectiveness, efficiency, and safety [6]. Evidence even suggests that, for patients, patient-centeredness may take precedence over pregnancy rates [7]. The use of ARTs in infertility treatment has increased substantially in recent years [2]. ARTs, including In-Vitro Fertilization (IVF), enable infertile couples to achieve pregnancy but are often associated with physical discomfort and emotional distress, including fatigue, depression, hopelessness, and anger [8]. Since ARTs can be highly stressful, complex, and demanding for both men and women, many infertile couples perceive them as psychologically burdensome. This emotional distress can be a major reason for treatment discontinuation or non-adherence to treatment [9-11]. Accordingly, infertile individuals require effective, high-quality, and patient-centered interventions [9, 10].
Monitoring patient-centeredness by assessing patients’ experiences with questionnaires is a valuable method for improving fertility care [12]. Selecting an appropriate tool that ensures valid and reliable measurements, is time- and cost-effective, and easy to implement is essential in any research [13], including the assessment of QPP among IVF patients. However, most existing tools are general rather than specific; some suffer from methodological limitations, and others are designed exclusively to assess women’s perspectives [14, 15]. There is a valid questionnaire for assessing the quality of care from the patient’s perspective in an IVF program (QPP-IVF) developed by Holter et al. in Sweden [14]. It is the first questionnaire specifically designed to assess infertile patients ‘ perspectives (for both men and women) regarding the quality of care, the importance of treatment aspects, and perceived care during IVF. Given that there is no Persian version of this questionnaire to assess QPP among IVF patients in Iran, this study aimed to translate and validate the Persian version of the modified QPP-IVF questionnaire.
Materials and Methods
In this methodological study, conducted from September 2020 to May 2021, participants were 200 infertile patients (142 women and 58 men) attending a specialized educational, clinical, and research infertility clinic in Rasht, northern Iran, who were recruited using a consecutive sampling method. The sample size required for conducting Exploratory Factor Analysis (EFA) is generally recommended by many researchers to be at least 100–250 [16]. In the present study, the sample size required for conducting EFA using Principal Component Analysis (PCA) was set at 142 samples. Inclusion criteria were willingness to participate in the study, infertility, undergoing IVF or Intracytoplasmic Sperm Injection (ICSI) at the stage between embryo transfer and pregnancy testing, Iranian nationality, and no use of psychotropic medications (based on self-report and medical records). Incomplete responses to the questionnaire were considered a non-inclusion criterion for the study. A sociodemographic/fertility form was used to survey their age, place of residence, educational level, number of IVF cycles, and type of oocyte received during treatment.
The QPP-IVF questionnaire assesses perceived quality of care (care received) and the subjective importance of various treatment aspects (importance of care from infertile patients’ perspectives), across four dimensions: a) medical-technical competence including three factors of medical care (one item), pain relief and physical care (two items), and waiting time (two items); b) physical-technical conditions including one factor of care room characteristics (three items); c) identity-oriented approach including five factors of information during treatment (three items), information after treatment (two items), participation (two items), responsibility/continuity (four items), and staff respect/commitment/empathy (six items); d) socio-cultural atmosphere including one factor of atmosphere and environment (four items). There was also another subscale (factor) that did not fit into any of the four dimensions, called “availability”, with two items. To assess perceived quality of care (perceived reality), each item was linked to the following statement: “This is what I experienced.” Responses to the items in the perceived quality of care section were measured on a four-point scale: 1 (disagree), 2 (partly agree), 3 (mostly agree), and 4 (completely agree). Higher scores indicate better care quality. For evaluating the subjective importance of various treatment aspects (from the patients’ perspective), each item was related to the statement: “This is how important it was to me.” Responses were graded on a four-point scale: 1 (of little or no importance), 2 (of some importance), 3 (of high importance), and 4 (of the highest importance). Higher scores indicate greater importance from the patients’ perspective. No total score was derived for the tool. There were also 12 supplementary (additional) questions (yes/no, multiple-choice, and open-ended) assessing patient satisfaction with the infertility treatment center. After consulting the developer, these were refined to 5 items. Therefore, the QPP-IVF comprised 31 gender-specific items (for women and men) and 5 supplementary questions. The items for men mirrored those for women, with one exception: the item “I had access to a comfortable environment during egg retrieval” was replaced with “I had access to a comfortable environment during sperm collection.” Additionally, where contextually appropriate, the pronoun “I” was substituted with “we” or “my wife” in the male version.
In the first phase of the study, the QPP-IVF questionnaire was translated into Persian after obtaining permission from its developer. The translation process was according to Wild et al.’s model [17], as outlined below: First, the original questionnaire was independently translated into Persian by two bilingual translators (faculty members specializing in reproductive health). The two translations were then merged into a single Persian draft, which was back-translated into English by an independent bilingual translator. After final approval and minor revisions suggested by the developer, the Persian version was finalized through item-by-item comparison of the Persian and English versions in coordination with the research team and a bilingual expert. In the second phase, the psychometric properties of the Persian version of the QPP-IVF were evaluated, including face validity, content validity, construct validity, convergent validity, discriminant validity, and reliability.
Face validity was first examined qualitatively by obtaining feedback from 10 infertile men and women regarding the relevance, clarity, ambiguity, and logical sequencing of the items. Quantitative face validity was then assessed by calculating item impact scores, based on responses from 20 infertile patients undergoing IVF (10 men and 10 women), who rated the importance of each item on a 5-point Likert scale (from 1=not important to 5=extremely important).
For qualitative content validity, the questionnaire was evaluated by 12 experts in midwifery, reproductive health, obstetrics, and instrument development. They assessed the items with respect to grammar, wording, relevance, and placement. Their suggestions were reviewed and incorporated by the research team. For quantitative content validity, both the Content Validity Ratio (CVR) and the Content Validity Index (CVI) were calculated. To determine the CVR, the experts were asked to rate the necessity of each item on a 3-point Likert scale (1=not necessary, 2=useful but not essential, 3=essential). The obtained values were then compared with the minimum acceptable CVR (0.56 for 12 experts) based on Lawshe’s table [18]. Items with CVR values exceeding this threshold were considered essential. The CVI was then calculated to assess the relevance of each item using a 4-point scale (1=not relevant, 2=somewhat relevant, 3=relevant, 4=highly relevant). Items with CVI scores above 0.79 were retained, those between 0.70 and 0.79 were revised, and those with scores below 0.70 were eliminated.
Prior to conducting a PCA for construct validity assessment, data distribution, missing values, and outliers were examined. Normality was assessed using skewness (±3) and kurtosis (±7). No questionnaires were excluded, and there were no missing data. An EFA using PCA was then conducted to assess construct validity. PCA was performed on data obtained from 142 completed questionnaires. The stability and consistency of the factor structure were evaluated using Promax rotation. The factors were then validated for both men and women. Additionally, the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were calculated. Analyses were conducted in SPSS software, version 16. Given that the majority of participants were women, all of whom underwent IVF, and in line with Holter et al.’s study [14], the analyses were conducted based on data from this group. To generalize the findings to the male version of the questionnaire and to the perceived reality section for both men and women, appropriate statistical indices, as well as reliability and validity coefficients, were utilized.
The Persian version of the Fertility Quality of Life Questionnaire (FertiQoL), whose reliability and validity were previously confirmed in the study by Maroufizadeh et al. [19], was used to assess convergent validity. The FertiQoL consists of two sections: The core section (core FertiQoL) and the treatment section (treatment FertiQoL). This study employed the treatment section of the FertiQoL questionnaire, which comprises 10 items evaluating two subscales: Environment (6 items) and Tolerability (4 items), rated on a 5-point Likert scale. Both Persian versions of the QPP-IVF and treatment FertiQoL questionnaires were administered to 50 women undergoing IVF treatment. Convergent validity was assessed using Pearson’s correlation coefficient between their scores.
To assess divergent validity, the World Health Organization (WHO) health status questionnaire was employed [20]. This questionnaire is a valid and widely used tool in health research, frequently employed to assess divergent validity in psychometric and clinical studies. The validity and reliability of the Persian version of this questionnaire were confirmed by Khalili et al. [21]. This 10-question, single-factor questionnaire assesses an individual’s health status across eight domains: Mobility, self-care, cognition, interpersonal relationships, vision, sleep, energy, and affect. Each domain is evaluated with one question, except for the vision domain, which includes two questions. Responses are recorded on a Likert scale: 1) none, 2) mild, 3) moderate, 4) severe, 5) extreme. The Persian versions of both the WHO individual questionnaire and the QPP-IVF were administered to 50 women undergoing IVF treatment (the same as those who participated in the convergent validity assessment phase). Following completion, divergent validity was established by calculating the correlation coefficient between the scores of the two questionnaires.
Reliability was assessed using two methods: internal consistency and test, re-test reliability. Internal consistency was evaluated by calculating cronbach’s α coefficient, with values above 0.6 considered acceptable [14]. For test-retest reliability, the questionnaire was administered twice at a two-week interval to 30 infertile patients (15 women and 15 men) undergoing IVF treatment. Participants were contacted by phone after two weeks for follow-up. Some completed the questionnaire in person, while others responded through a phone interview. The Intraclass Correlation Coefficient (ICC) was then calculated to assess test-retest reliability.
Results
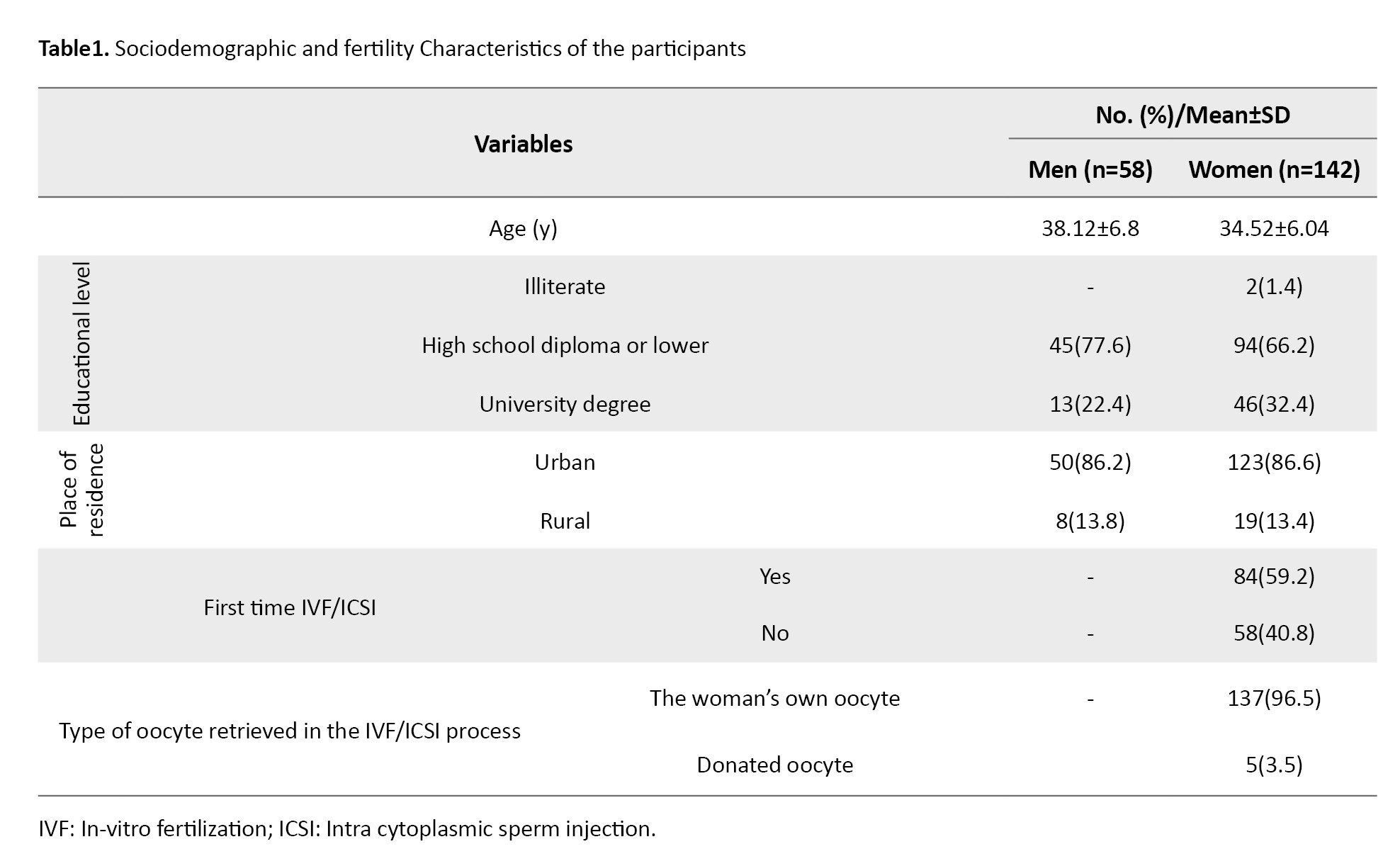
Among 200 infertile patients, the mean age of women (n=142) was 34.52±6.04 years, and the mean age of men (n=58) was 38.12±6.8 years. Most women (86.6%) and men (86.2%) resided in urban areas. Most women (66.2%) and men (77.6%) had a high school diploma or lower education. Embryo transfer was performed for the first time in 59.2% of women. In 96.5% of these cases, embryos originated from their own oocytes and their spouses’ sperm (Table 1).
The quantitative face validity assessment of the QPP-IVF indicated item impact scores ranging from 1.5 to 5 for both female and male versions. The CVR values ranging from 0.66 to 1 demonstrated that all items in both female and male versions were acceptable. The CVI values were above 0.79 for all items, ranging from 0.83 to 1 across all items of both female and male versions. The overall scale CVI (S-CVI/Ave) was estimated at 0.92. Accordingly, all items were retained at this stage.
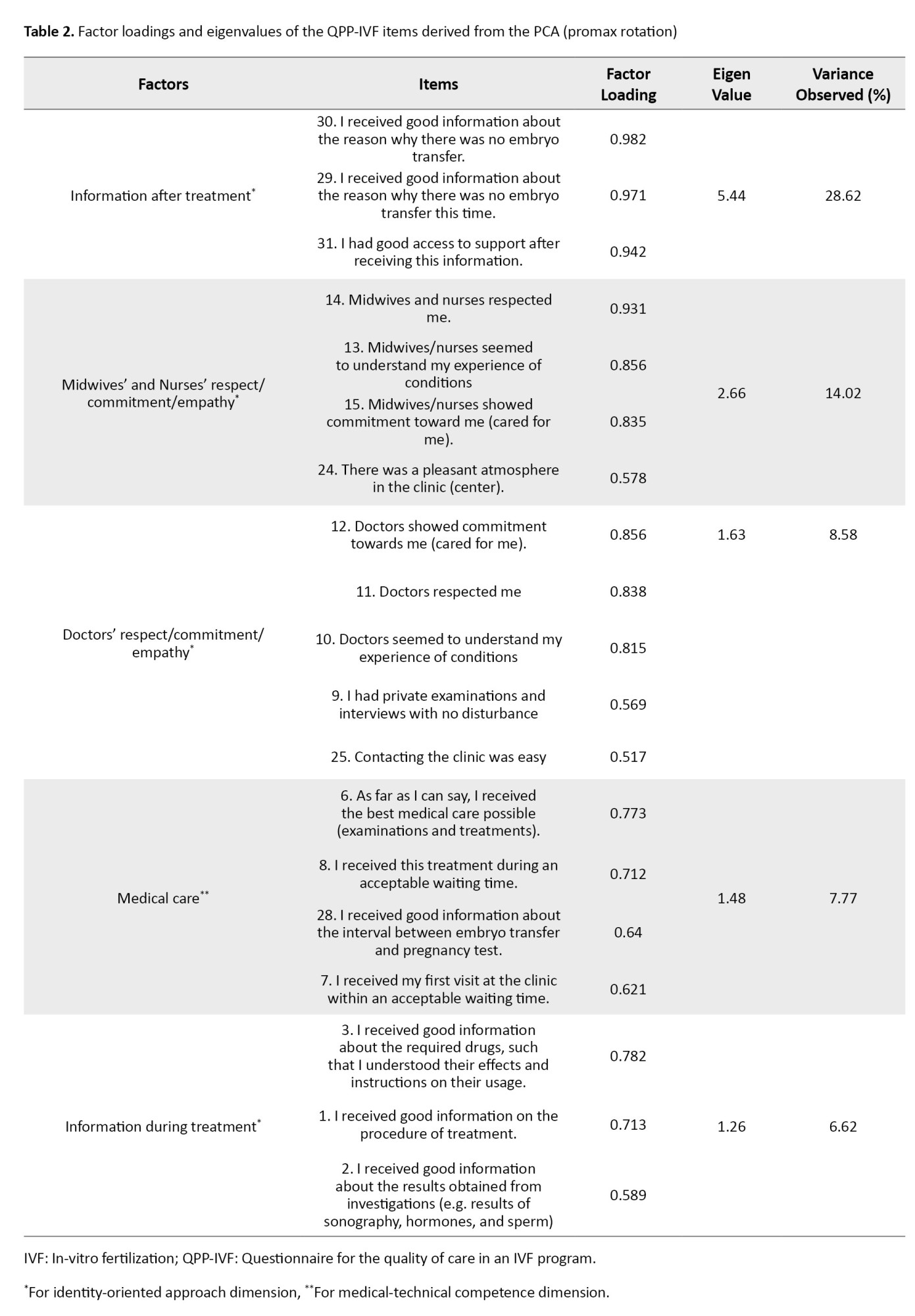
Before conducting the PCA, the mean score of item 20 (“I had good opportunities to see a psychologist/counselor if I needed”) was lower than that of the other items based on participants’ responses; therefore, this item was removed. In the preliminary assessment of data normality, items 4 (“I know which doctor is responsible for my treatment”), 16 (“I had access to a pleasant room while waiting for oocyte aspiration”), 17 (“I had access to a pleasant treatment room during oocyte aspiration and embryo transfer”), 18 (“I received effective pain relief during oocyte aspiration”), 19 (“I received the best possible physical care during oocyte aspiration, as far as I can tell”), 26 (“It was easy to get an appointment at the clinic”), and 27 (“I received good information regarding the fertilization and embryo development at the time of embryo transfer”) did not meet the assumption of normal distribution. Consequently, these items were excluded from factor analysis. Overall, out of 31 items, 8 were eliminated at this stage. The KMO value was 0.765, and Bartlett’s test was significant (χ2=1504.555, P<0.001), confirming the suitability of the data for factor analysis. Accordingly, PCA was performed on the 23 remaining items using Promax rotation. The number of factors was determined based on eigenvalues (>1) and the scree plot (Figure 1). Five factors with eigenvalues greater than one (5.44, 2.66, 1.63, 1.48, and 1.26) were extracted, explaining 65.61% of the variance. The criterion for item retention was a factor loading above 0.50. Accordingly, out of the 23 items, Item 5 (“I know which midwife or nurse is responsible for my care”), Item 21 (“I had good opportunities to participate in the decisions that applied to my treatment”), Item 22 (“My treatment was determined by my needs rather than the staff’s routines”), and Item 23 (“My partner was treated well”) were excluded due to factor loadings <0.50. Ultimately, 19 items remained, grouped into five factors: Information after treatment (items 29-31), midwives’ and nurses’ respect/commitment/empathy (items 13-15 and 24), doctors’ respect/commitment/empathy (items 9-12 and 25), medical care (items 6-8, and 28), and information during treatment (items 1-3). These five factors were categorized into two dimensions: Identity-oriented approach and medical-technical competence (Table 2).
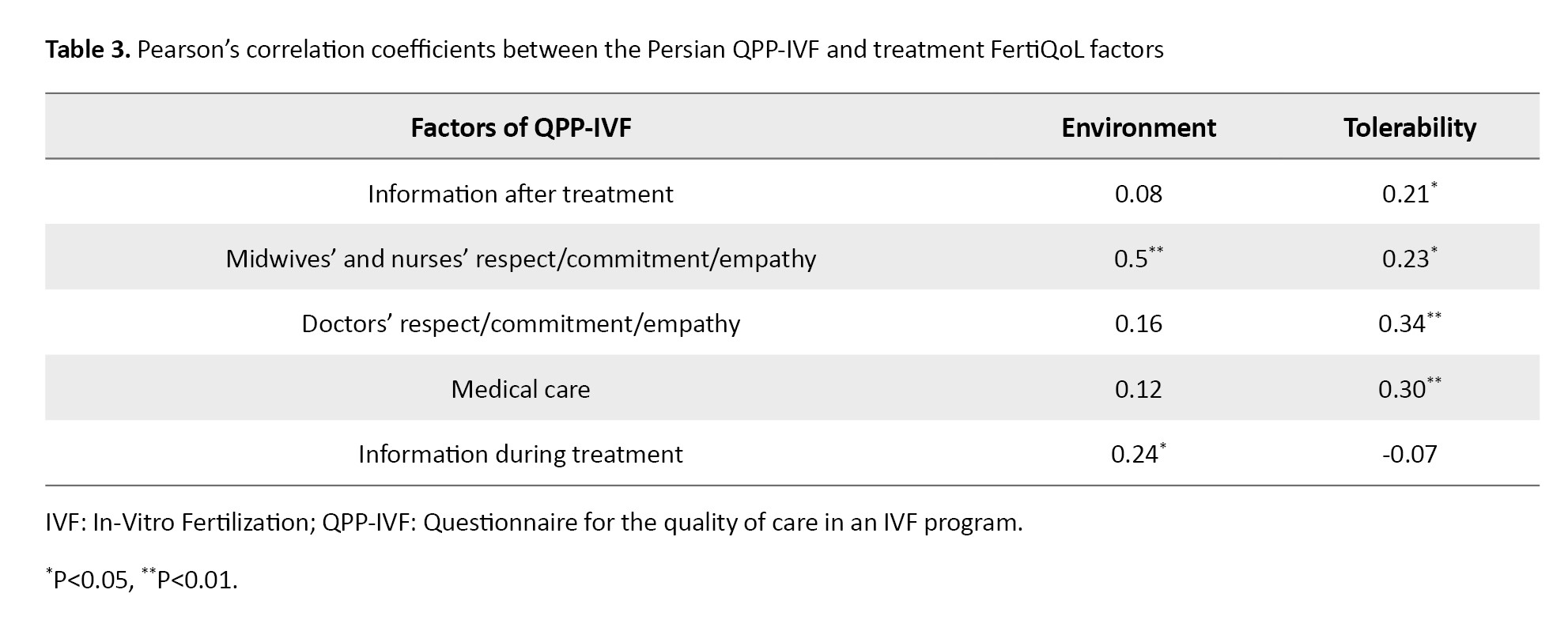
In assessing convergent validity, Pearson’s correlation coefficients revealed a significant correlation between Factor 1 of the QPP-IVF (information after treatment) and the treatment tolerability factor of the FertiQoL (r=0.21, P=0.040). Factor 2 (midwives’ and nurses’ respect/commitment/empathy) demonstrated significant positive correlations with both treatment environment (r=0.50, P=0.001) and treatment tolerability (r=0.23, P=0.023) factors of FertiQoL. Similarly, factor 3 (doctors’ respect/commitment/empathy) showed a significant correlation with treatment tolerability (r=0.34, P=0.001). Factor 4 (medical care) was significantly correlated with treatment tolerability (r=0.30, P=0.003). Finally, factor 5 (information during treatment) exhibited a significant correlation with the treatment environment (r=0.24, P=0.016). These results are summarized in Table 3.
In assessing divergent validity, Pearson’s correlation analysis revealed no statistically significant correlation between the scores of the Persian versions of the QPP-IVF and the WHO health status questionnaire (P>0.05).
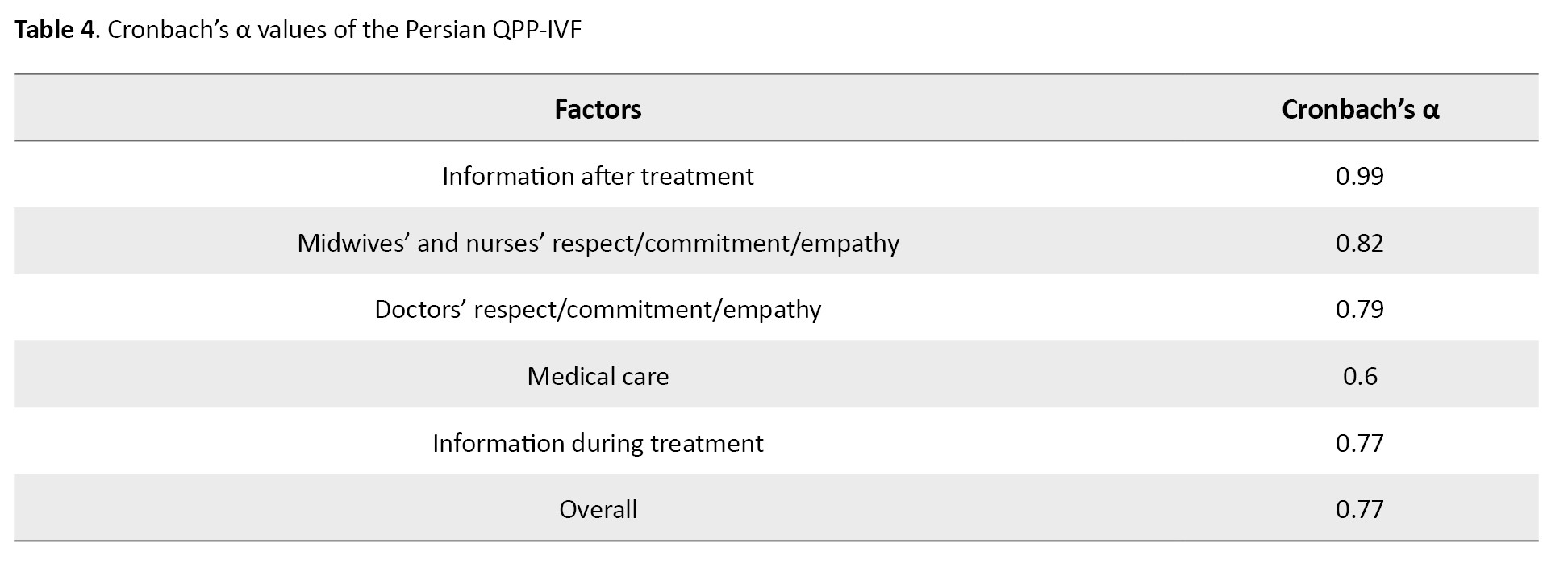
In assessing the internal consistency of the 19-item Persian QPP-IVF questionnaire, cronbach’s α values ranged from 0.6 to 0.99 for the five factors and 0.77 for the entire questionnaire (Table 4).
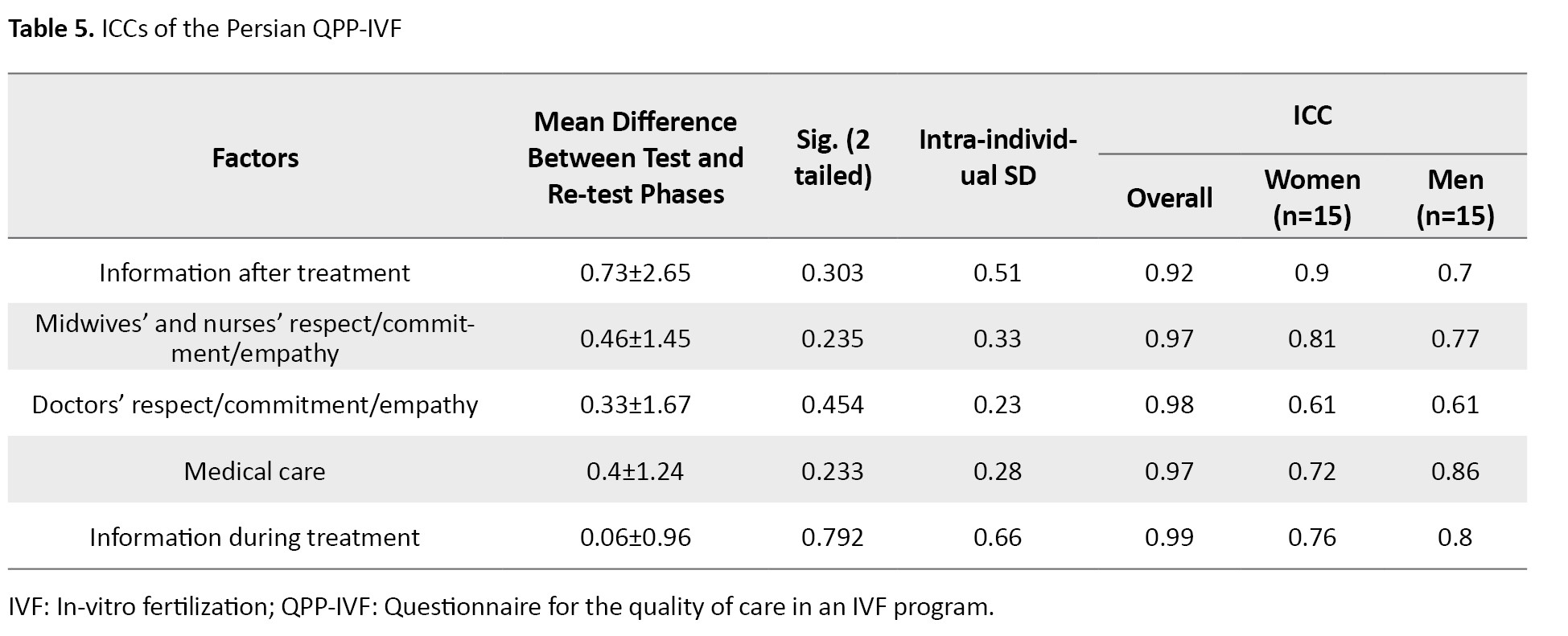
Furthermore, the results of the test–retest reliability (with a two-week interval) showed that the ICC values of factors 1, 2, 4, and 5 ranged from 0.72 to 0.9 for women and from 0.7 to 0.86 for men. Factor 3 had moderate reliability (ICC=0.61) for both genders. The overall test–retest reliability was high, ranging between 0.92 and 0.99, confirming the strong test–retest reliability of the Persian QPP-IVF (Table 5).
Consistent with Holter et al. [14], the five additional questions at the end of the QPP-IVF questionnaire were excluded from the factor analysis. In order to evaluate these questions, the discrimination coefficient between these questions (5 questions) and the questionnaire factors was calculated separately in the questionnaires for women and men, and the results showed that the discrimination coefficient was between zero and +1, which is desirable.
Discussion
The purpose of this study was to determine the psychometric properties of the Persian version of the modified QPP-IVF. The findings demonstrated that the Persian QPP-IVF had satisfactory validity (face, content, and construct validity) and reliability (internal consistency and test-retest reliability).
Based on the EFA results with Promax rotation, 19 items clustered into five factors, accounting for 65.61% of the variance, which were subsequently grouped into two overarching dimensions: Identity-oriented approach and medical–technical competence. The first dimension included the factors of information after treatment, midwives’ and nurses’ respect/commitment/empathy, doctors’ respect/commitment/empathy, and information during treatment, whereas the second dimension included the medical care factor. This factor structure differs from that of the original version of the questionnaire [14], which has 11 factors grouped into four dimensions. This discrepancy can be due to differences in participants’ demographic characteristics, sampling methods, sample size, research settings, data analysis techniques, fertility service approaches, and socio-cultural contexts. Sandsdalen et al. [22] also developed a short form of the QPP for Palliative Care questionnaire, highlighting the feasibility of a more concise yet valid instrument.
Pearson’s correlation test results revealed statistically significant associations between some factors of the QPP-IVF and treatment FertiQoL. Therefore, the Persian version of the QPP-IVF demonstrated acceptable convergent validity. This is consistent with the results reported by Holter et al. for the original version [14]. Moreover, Pearson’s correlation test results indicated no statistically significant correlations between the Persian versions of the QPP-IVF and the WHO health status questionnaire. These findings suggest that the Persian instrument has divergent validity, indicating that it targets only the concept it was intended to measure, rather than individual health status.
The overall cronbach’s α for the instrument was high, indicating high internal consistency of the entire questionnaire. For the five factors, internal consistency was acceptable, although factor 4 (medical care) demonstrated a comparatively lower consistency. Considering the importance of the items within this factor and based on expert judgment, this factor was retained [13]. Test–retest reliability of the instrument was also evaluated at a two-week interval. All factors had ICC values within the desirable range for both men and women, consistent with the findings of Holter et al. [14].
The QPP-IVF can assess both the subjective importance of treatment aspects from the patient’s perspective and the perceived quality of care during an IVF program. This dual assessment makes it a valuable tool for identifying areas that require improvement and recognizing aspects that patients perceive as satisfactory. The study had some limitations, including the absence of Confirmatory Factor Analysis (CFA) in assessing construct validity, and small sample size of men compared to women due to their limited availability and participation during the infertility treatment process. Future studies should conduct CFA to validate the questionnaire’s factorial structure, evaluate its psychometric properties in larger male samples, and include other infertility treatment centers in Iran to enhance the generalizability of the results.
Overall, it can be concluded that the 19-item Persian version of the QPP-IVF has acceptable and satisfactory psychometric properties. It is adaptable to the Iranian culture, easy to understand and use by individuals experiencing infertility in Iran, and applicable in both clinical and research settings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1399.186), and was conducted in accordance with the ethical principles of the 1964 Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
Funding
This study was extracted from the master’s thesis of the Maryam Borjalizadeh, approved by the Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences.
Authors' contributions
Study design and draft preparation: Maryam Borjalizadeh, Fatemeh Jafarzadeh-Kenarsari, Tahereh Seyednoori, and Ziba Zahiri Sorouri; Data collection: Maryam Borjalizadeh; Statistical analysis: Ehsan Kazemnezhad Leyli; Data analysis, interpretation, review and editing: Maryam Borjalizadeh, Fatemeh Jafarzadeh-Kenarsari, and Tahereh Seyednoori; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all patients who participated in this study for their cooperation.
References:
Quality of care refers to the degree to which the healthcare services provided to patients can enhance the desired health outcomes. To achieve quality care, healthcare services should be safe, effective, timely, efficient, equitable, integrated, needs-based, and patient-centered [1]. Focusing on patients’ values and needs (patient-centered care) is a key element in improving the quality of care [2, 3]. Patients’ perceptions are a valuable source of information for planning and evaluation of healthcare services [4]. Quality from Patients’ Perspective (QPP) can also serve as a measure of patients’ experiences with the services received and their expectations of the provided care [5].
In the field of Assisted Reproductive Technologies (ARTs), patient-centeredness is a critical dimension of high-quality care, alongside effectiveness, efficiency, and safety [6]. Evidence even suggests that, for patients, patient-centeredness may take precedence over pregnancy rates [7]. The use of ARTs in infertility treatment has increased substantially in recent years [2]. ARTs, including In-Vitro Fertilization (IVF), enable infertile couples to achieve pregnancy but are often associated with physical discomfort and emotional distress, including fatigue, depression, hopelessness, and anger [8]. Since ARTs can be highly stressful, complex, and demanding for both men and women, many infertile couples perceive them as psychologically burdensome. This emotional distress can be a major reason for treatment discontinuation or non-adherence to treatment [9-11]. Accordingly, infertile individuals require effective, high-quality, and patient-centered interventions [9, 10].
Monitoring patient-centeredness by assessing patients’ experiences with questionnaires is a valuable method for improving fertility care [12]. Selecting an appropriate tool that ensures valid and reliable measurements, is time- and cost-effective, and easy to implement is essential in any research [13], including the assessment of QPP among IVF patients. However, most existing tools are general rather than specific; some suffer from methodological limitations, and others are designed exclusively to assess women’s perspectives [14, 15]. There is a valid questionnaire for assessing the quality of care from the patient’s perspective in an IVF program (QPP-IVF) developed by Holter et al. in Sweden [14]. It is the first questionnaire specifically designed to assess infertile patients ‘ perspectives (for both men and women) regarding the quality of care, the importance of treatment aspects, and perceived care during IVF. Given that there is no Persian version of this questionnaire to assess QPP among IVF patients in Iran, this study aimed to translate and validate the Persian version of the modified QPP-IVF questionnaire.
Materials and Methods
In this methodological study, conducted from September 2020 to May 2021, participants were 200 infertile patients (142 women and 58 men) attending a specialized educational, clinical, and research infertility clinic in Rasht, northern Iran, who were recruited using a consecutive sampling method. The sample size required for conducting Exploratory Factor Analysis (EFA) is generally recommended by many researchers to be at least 100–250 [16]. In the present study, the sample size required for conducting EFA using Principal Component Analysis (PCA) was set at 142 samples. Inclusion criteria were willingness to participate in the study, infertility, undergoing IVF or Intracytoplasmic Sperm Injection (ICSI) at the stage between embryo transfer and pregnancy testing, Iranian nationality, and no use of psychotropic medications (based on self-report and medical records). Incomplete responses to the questionnaire were considered a non-inclusion criterion for the study. A sociodemographic/fertility form was used to survey their age, place of residence, educational level, number of IVF cycles, and type of oocyte received during treatment.
The QPP-IVF questionnaire assesses perceived quality of care (care received) and the subjective importance of various treatment aspects (importance of care from infertile patients’ perspectives), across four dimensions: a) medical-technical competence including three factors of medical care (one item), pain relief and physical care (two items), and waiting time (two items); b) physical-technical conditions including one factor of care room characteristics (three items); c) identity-oriented approach including five factors of information during treatment (three items), information after treatment (two items), participation (two items), responsibility/continuity (four items), and staff respect/commitment/empathy (six items); d) socio-cultural atmosphere including one factor of atmosphere and environment (four items). There was also another subscale (factor) that did not fit into any of the four dimensions, called “availability”, with two items. To assess perceived quality of care (perceived reality), each item was linked to the following statement: “This is what I experienced.” Responses to the items in the perceived quality of care section were measured on a four-point scale: 1 (disagree), 2 (partly agree), 3 (mostly agree), and 4 (completely agree). Higher scores indicate better care quality. For evaluating the subjective importance of various treatment aspects (from the patients’ perspective), each item was related to the statement: “This is how important it was to me.” Responses were graded on a four-point scale: 1 (of little or no importance), 2 (of some importance), 3 (of high importance), and 4 (of the highest importance). Higher scores indicate greater importance from the patients’ perspective. No total score was derived for the tool. There were also 12 supplementary (additional) questions (yes/no, multiple-choice, and open-ended) assessing patient satisfaction with the infertility treatment center. After consulting the developer, these were refined to 5 items. Therefore, the QPP-IVF comprised 31 gender-specific items (for women and men) and 5 supplementary questions. The items for men mirrored those for women, with one exception: the item “I had access to a comfortable environment during egg retrieval” was replaced with “I had access to a comfortable environment during sperm collection.” Additionally, where contextually appropriate, the pronoun “I” was substituted with “we” or “my wife” in the male version.
In the first phase of the study, the QPP-IVF questionnaire was translated into Persian after obtaining permission from its developer. The translation process was according to Wild et al.’s model [17], as outlined below: First, the original questionnaire was independently translated into Persian by two bilingual translators (faculty members specializing in reproductive health). The two translations were then merged into a single Persian draft, which was back-translated into English by an independent bilingual translator. After final approval and minor revisions suggested by the developer, the Persian version was finalized through item-by-item comparison of the Persian and English versions in coordination with the research team and a bilingual expert. In the second phase, the psychometric properties of the Persian version of the QPP-IVF were evaluated, including face validity, content validity, construct validity, convergent validity, discriminant validity, and reliability.
Face validity was first examined qualitatively by obtaining feedback from 10 infertile men and women regarding the relevance, clarity, ambiguity, and logical sequencing of the items. Quantitative face validity was then assessed by calculating item impact scores, based on responses from 20 infertile patients undergoing IVF (10 men and 10 women), who rated the importance of each item on a 5-point Likert scale (from 1=not important to 5=extremely important).
For qualitative content validity, the questionnaire was evaluated by 12 experts in midwifery, reproductive health, obstetrics, and instrument development. They assessed the items with respect to grammar, wording, relevance, and placement. Their suggestions were reviewed and incorporated by the research team. For quantitative content validity, both the Content Validity Ratio (CVR) and the Content Validity Index (CVI) were calculated. To determine the CVR, the experts were asked to rate the necessity of each item on a 3-point Likert scale (1=not necessary, 2=useful but not essential, 3=essential). The obtained values were then compared with the minimum acceptable CVR (0.56 for 12 experts) based on Lawshe’s table [18]. Items with CVR values exceeding this threshold were considered essential. The CVI was then calculated to assess the relevance of each item using a 4-point scale (1=not relevant, 2=somewhat relevant, 3=relevant, 4=highly relevant). Items with CVI scores above 0.79 were retained, those between 0.70 and 0.79 were revised, and those with scores below 0.70 were eliminated.
Prior to conducting a PCA for construct validity assessment, data distribution, missing values, and outliers were examined. Normality was assessed using skewness (±3) and kurtosis (±7). No questionnaires were excluded, and there were no missing data. An EFA using PCA was then conducted to assess construct validity. PCA was performed on data obtained from 142 completed questionnaires. The stability and consistency of the factor structure were evaluated using Promax rotation. The factors were then validated for both men and women. Additionally, the Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were calculated. Analyses were conducted in SPSS software, version 16. Given that the majority of participants were women, all of whom underwent IVF, and in line with Holter et al.’s study [14], the analyses were conducted based on data from this group. To generalize the findings to the male version of the questionnaire and to the perceived reality section for both men and women, appropriate statistical indices, as well as reliability and validity coefficients, were utilized.
The Persian version of the Fertility Quality of Life Questionnaire (FertiQoL), whose reliability and validity were previously confirmed in the study by Maroufizadeh et al. [19], was used to assess convergent validity. The FertiQoL consists of two sections: The core section (core FertiQoL) and the treatment section (treatment FertiQoL). This study employed the treatment section of the FertiQoL questionnaire, which comprises 10 items evaluating two subscales: Environment (6 items) and Tolerability (4 items), rated on a 5-point Likert scale. Both Persian versions of the QPP-IVF and treatment FertiQoL questionnaires were administered to 50 women undergoing IVF treatment. Convergent validity was assessed using Pearson’s correlation coefficient between their scores.
To assess divergent validity, the World Health Organization (WHO) health status questionnaire was employed [20]. This questionnaire is a valid and widely used tool in health research, frequently employed to assess divergent validity in psychometric and clinical studies. The validity and reliability of the Persian version of this questionnaire were confirmed by Khalili et al. [21]. This 10-question, single-factor questionnaire assesses an individual’s health status across eight domains: Mobility, self-care, cognition, interpersonal relationships, vision, sleep, energy, and affect. Each domain is evaluated with one question, except for the vision domain, which includes two questions. Responses are recorded on a Likert scale: 1) none, 2) mild, 3) moderate, 4) severe, 5) extreme. The Persian versions of both the WHO individual questionnaire and the QPP-IVF were administered to 50 women undergoing IVF treatment (the same as those who participated in the convergent validity assessment phase). Following completion, divergent validity was established by calculating the correlation coefficient between the scores of the two questionnaires.
Reliability was assessed using two methods: internal consistency and test, re-test reliability. Internal consistency was evaluated by calculating cronbach’s α coefficient, with values above 0.6 considered acceptable [14]. For test-retest reliability, the questionnaire was administered twice at a two-week interval to 30 infertile patients (15 women and 15 men) undergoing IVF treatment. Participants were contacted by phone after two weeks for follow-up. Some completed the questionnaire in person, while others responded through a phone interview. The Intraclass Correlation Coefficient (ICC) was then calculated to assess test-retest reliability.
Results
Among 200 infertile patients, the mean age of women (n=142) was 34.52±6.04 years, and the mean age of men (n=58) was 38.12±6.8 years. Most women (86.6%) and men (86.2%) resided in urban areas. Most women (66.2%) and men (77.6%) had a high school diploma or lower education. Embryo transfer was performed for the first time in 59.2% of women. In 96.5% of these cases, embryos originated from their own oocytes and their spouses’ sperm (Table 1).

The quantitative face validity assessment of the QPP-IVF indicated item impact scores ranging from 1.5 to 5 for both female and male versions. The CVR values ranging from 0.66 to 1 demonstrated that all items in both female and male versions were acceptable. The CVI values were above 0.79 for all items, ranging from 0.83 to 1 across all items of both female and male versions. The overall scale CVI (S-CVI/Ave) was estimated at 0.92. Accordingly, all items were retained at this stage.
Before conducting the PCA, the mean score of item 20 (“I had good opportunities to see a psychologist/counselor if I needed”) was lower than that of the other items based on participants’ responses; therefore, this item was removed. In the preliminary assessment of data normality, items 4 (“I know which doctor is responsible for my treatment”), 16 (“I had access to a pleasant room while waiting for oocyte aspiration”), 17 (“I had access to a pleasant treatment room during oocyte aspiration and embryo transfer”), 18 (“I received effective pain relief during oocyte aspiration”), 19 (“I received the best possible physical care during oocyte aspiration, as far as I can tell”), 26 (“It was easy to get an appointment at the clinic”), and 27 (“I received good information regarding the fertilization and embryo development at the time of embryo transfer”) did not meet the assumption of normal distribution. Consequently, these items were excluded from factor analysis. Overall, out of 31 items, 8 were eliminated at this stage. The KMO value was 0.765, and Bartlett’s test was significant (χ2=1504.555, P<0.001), confirming the suitability of the data for factor analysis. Accordingly, PCA was performed on the 23 remaining items using Promax rotation. The number of factors was determined based on eigenvalues (>1) and the scree plot (Figure 1). Five factors with eigenvalues greater than one (5.44, 2.66, 1.63, 1.48, and 1.26) were extracted, explaining 65.61% of the variance. The criterion for item retention was a factor loading above 0.50. Accordingly, out of the 23 items, Item 5 (“I know which midwife or nurse is responsible for my care”), Item 21 (“I had good opportunities to participate in the decisions that applied to my treatment”), Item 22 (“My treatment was determined by my needs rather than the staff’s routines”), and Item 23 (“My partner was treated well”) were excluded due to factor loadings <0.50. Ultimately, 19 items remained, grouped into five factors: Information after treatment (items 29-31), midwives’ and nurses’ respect/commitment/empathy (items 13-15 and 24), doctors’ respect/commitment/empathy (items 9-12 and 25), medical care (items 6-8, and 28), and information during treatment (items 1-3). These five factors were categorized into two dimensions: Identity-oriented approach and medical-technical competence (Table 2).

In assessing convergent validity, Pearson’s correlation coefficients revealed a significant correlation between Factor 1 of the QPP-IVF (information after treatment) and the treatment tolerability factor of the FertiQoL (r=0.21, P=0.040). Factor 2 (midwives’ and nurses’ respect/commitment/empathy) demonstrated significant positive correlations with both treatment environment (r=0.50, P=0.001) and treatment tolerability (r=0.23, P=0.023) factors of FertiQoL. Similarly, factor 3 (doctors’ respect/commitment/empathy) showed a significant correlation with treatment tolerability (r=0.34, P=0.001). Factor 4 (medical care) was significantly correlated with treatment tolerability (r=0.30, P=0.003). Finally, factor 5 (information during treatment) exhibited a significant correlation with the treatment environment (r=0.24, P=0.016). These results are summarized in Table 3.

In assessing divergent validity, Pearson’s correlation analysis revealed no statistically significant correlation between the scores of the Persian versions of the QPP-IVF and the WHO health status questionnaire (P>0.05).
In assessing the internal consistency of the 19-item Persian QPP-IVF questionnaire, cronbach’s α values ranged from 0.6 to 0.99 for the five factors and 0.77 for the entire questionnaire (Table 4).

Furthermore, the results of the test–retest reliability (with a two-week interval) showed that the ICC values of factors 1, 2, 4, and 5 ranged from 0.72 to 0.9 for women and from 0.7 to 0.86 for men. Factor 3 had moderate reliability (ICC=0.61) for both genders. The overall test–retest reliability was high, ranging between 0.92 and 0.99, confirming the strong test–retest reliability of the Persian QPP-IVF (Table 5).

Consistent with Holter et al. [14], the five additional questions at the end of the QPP-IVF questionnaire were excluded from the factor analysis. In order to evaluate these questions, the discrimination coefficient between these questions (5 questions) and the questionnaire factors was calculated separately in the questionnaires for women and men, and the results showed that the discrimination coefficient was between zero and +1, which is desirable.
Discussion
The purpose of this study was to determine the psychometric properties of the Persian version of the modified QPP-IVF. The findings demonstrated that the Persian QPP-IVF had satisfactory validity (face, content, and construct validity) and reliability (internal consistency and test-retest reliability).
Based on the EFA results with Promax rotation, 19 items clustered into five factors, accounting for 65.61% of the variance, which were subsequently grouped into two overarching dimensions: Identity-oriented approach and medical–technical competence. The first dimension included the factors of information after treatment, midwives’ and nurses’ respect/commitment/empathy, doctors’ respect/commitment/empathy, and information during treatment, whereas the second dimension included the medical care factor. This factor structure differs from that of the original version of the questionnaire [14], which has 11 factors grouped into four dimensions. This discrepancy can be due to differences in participants’ demographic characteristics, sampling methods, sample size, research settings, data analysis techniques, fertility service approaches, and socio-cultural contexts. Sandsdalen et al. [22] also developed a short form of the QPP for Palliative Care questionnaire, highlighting the feasibility of a more concise yet valid instrument.
Pearson’s correlation test results revealed statistically significant associations between some factors of the QPP-IVF and treatment FertiQoL. Therefore, the Persian version of the QPP-IVF demonstrated acceptable convergent validity. This is consistent with the results reported by Holter et al. for the original version [14]. Moreover, Pearson’s correlation test results indicated no statistically significant correlations between the Persian versions of the QPP-IVF and the WHO health status questionnaire. These findings suggest that the Persian instrument has divergent validity, indicating that it targets only the concept it was intended to measure, rather than individual health status.
The overall cronbach’s α for the instrument was high, indicating high internal consistency of the entire questionnaire. For the five factors, internal consistency was acceptable, although factor 4 (medical care) demonstrated a comparatively lower consistency. Considering the importance of the items within this factor and based on expert judgment, this factor was retained [13]. Test–retest reliability of the instrument was also evaluated at a two-week interval. All factors had ICC values within the desirable range for both men and women, consistent with the findings of Holter et al. [14].
The QPP-IVF can assess both the subjective importance of treatment aspects from the patient’s perspective and the perceived quality of care during an IVF program. This dual assessment makes it a valuable tool for identifying areas that require improvement and recognizing aspects that patients perceive as satisfactory. The study had some limitations, including the absence of Confirmatory Factor Analysis (CFA) in assessing construct validity, and small sample size of men compared to women due to their limited availability and participation during the infertility treatment process. Future studies should conduct CFA to validate the questionnaire’s factorial structure, evaluate its psychometric properties in larger male samples, and include other infertility treatment centers in Iran to enhance the generalizability of the results.
Overall, it can be concluded that the 19-item Persian version of the QPP-IVF has acceptable and satisfactory psychometric properties. It is adaptable to the Iranian culture, easy to understand and use by individuals experiencing infertility in Iran, and applicable in both clinical and research settings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR.GUMS.REC.1399.186), and was conducted in accordance with the ethical principles of the 1964 Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
Funding
This study was extracted from the master’s thesis of the Maryam Borjalizadeh, approved by the Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences.
Authors' contributions
Study design and draft preparation: Maryam Borjalizadeh, Fatemeh Jafarzadeh-Kenarsari, Tahereh Seyednoori, and Ziba Zahiri Sorouri; Data collection: Maryam Borjalizadeh; Statistical analysis: Ehsan Kazemnezhad Leyli; Data analysis, interpretation, review and editing: Maryam Borjalizadeh, Fatemeh Jafarzadeh-Kenarsari, and Tahereh Seyednoori; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank all patients who participated in this study for their cooperation.
References:
- Marie-Paule K, Grant TE, Stefano S, Edward TK, Niek K, Ian F et al. Delivering quality health services: A global imperative for universal health coverage (English). Washington: World Bank Group; 2018. [Link]
- Borghi L, Menichetti J, Vegni E. Editorial: Patient-centered infertility care: Current research and future perspectives on psychosocial, relational, and communication aspects. Front Psychol. 2021; 12:712485. [DOI:10.3389/fpsyg.2021.712485] [PMID]
- Le Thi Thao H, Thi Trang N, Thanh Hien TT, Thi Sen T. Quality of care from the patient’s perspective and related factors. Br J Nurs Stud. 2024; 4(2):76-81. [DOI:10.32996/bjns.2024.4.2.9]
- Karaca A, Durna Z. Patient satisfaction with the quality of nursing care. Nurs Open. 2019; 6(2):535-45. [DOI:10.1002/nop2.237] [PMID]
- Wilde B, Starrin B, Larsson G, Larsson M. Quality of care from a patient perspective--a grounded theory study. Scand J Caring Sci. 1993; 7(2):113-20. [DOI:10.1111/j.1471-6712.1993.tb00180.x] [PMID]
- Gameiro S. Patient-centered ivf care. In: Domar AD, Sakkas D, Toth TL, editors. Patient-centered assisted reproduction: How to Integrate Exceptional Care with Cutting-Edge Technology. Cambridge: Cambridge University Press. 2020; 156-69. [DOI:10.1017/9781108859486.013]
- Shandley LM, Hipp HS, Anderson-Bialis J, Anderson-Bialis D, Boulet SL, McKenzie LJ, et al. Patient-centered care: Factors associated with reporting a positive experience at United States fertility clinics. Fertil Steril. 2020; 113(4):797-810. [DOI:10.1016/j.fertnstert.2019.12.040] [PMID]
- Gonen LD, Bokek-Cohen Y. Valuing the invaluable: Do emotional experiences during fertility treatments affect the willingness to pay for them? Eur Rev Appl Psychol. 2018; 68(2):45-60. [DOI:10.1016/j.erap.2018.01.002]
- Webair HH, Ismail TAT, Ismail SB, Mohd Noor N. Patient-centred infertility care: A scoping review protocol. BMJ Open. 2019; 9(11):e032266. [DOI:10.1136/bmjopen-2019-032266] [PMID]
- van Empel IW, Aarts JW, Cohlen BJ, Huppelschoten DA, Laven JS, Nelen WL, et al. Measuring patient-centredness, the neglected outcome in fertility care: A random multicentre validation study. Hum Reprod. 2010; 25(10):2516-26. [DOI:10.1093/humrep/deq219] [PMID]
- Toftager M, Sylvest R, Schmidt L, Bogstad J, Løssl K, Prætorius L, et al. Quality of life and psychosocial and physical well-being among 1,023 women during their first assisted reproductive technology treatment: Secondary outcome to a randomized controlled trial comparing gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist protocols. Fertil Steril. 2018; 109(1):154-64. [DOI:10.1016/j.fertnstert.2017.09.020] [PMID]
- van der Kolk L, Smit E, Bloemer J, van Wijk LM. The PCQ-infertility revised: A new digital instrument to measure treatment satisfaction of fertility patients. Patient Relat Outcome Meas. 2023; 14:223-34. [DOI:10.2147/PROM.S416182] [PMID]
- Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. Philadelphia: Wolters Kluwer; 2017. [Link]
- Holter H, Sandin-Bojö AK, Gejervall AL, Wikland M, Wilde-Larsson B, Bergh C. Quality of care in an IVF programme from a patient's perspective: Development of a validated instrument. Hum Reprod. 2014; 29(3):534-47. [DOI:10.1093/humrep/det421] [PMID]
- Mourad SM, Curtis C, Gudex G, Merrilees M, Peek J, Sadler L. Measuring patient-centredness in publicly funded fertility care: A New Zealand validation and international comparison of the Patient-Centred Questionnaire-Infertility. Aust N Z J Obstet Gynaecol. 2019; 59(2):265-71. [DOI:10.1111/ajo.12869] [PMID]
- Kyriazos T. Applied psychometrics: Sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. Psychology. 2018; 9(8):2207-30. [DOI:10.4236/psych.2018.98126]
- Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A,et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: Report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005; 8(2):94-104. [DOI:10.1111/j.1524-4733.2005.04054.x] [PMID]
- Lawshe CH. A quantitative approach to content validity 1. Perspsychol. 1975; 28(4):563-75. [DOI:10.1111/j.1744-6570.1975.tb01393.x]
- Maroufizadeh S, Ghaheri A, Amini P, Samani RO. Psychometric properties of the fertility quality of life instrument in Iranian infertile women. Int J Fertil Steril. 2016; 10(4):371-9. [DOI:10.22074/ijfs.2016.4696] [PMID]
- World Health Organization (WHO): Health systems performance assessment: Debates, methods and empiricism. Geneva: WHO; 2003. [Link]
- Khalili F, Nejat S, Baygi V, Yadegarfar G, Yazdani K, Kazem M. [Persian version of world health surveys individual questionnaire: A validation study (Persian)]. J Med Counc Islam Repub Iran. 2016; 34(3):201-8. [Link]
- Sandsdalen T, Grøndahl VA, Wilde-Larsson B. Development of a short form of the questionnaire quality from the patient's perspective for palliative care (QPP-PC). J Multidiscip Healthc. 2020; 13:495-506. [DOI:10.2147/JMDH.S246184] [PMID]
Article Type : Research |
Subject:
General
Received: 2022/02/15 | Accepted: 2025/09/8 | Published: 2025/09/8
Received: 2022/02/15 | Accepted: 2025/09/8 | Published: 2025/09/8
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




