Tue, Feb 3, 2026
Volume 31, Issue 1 (12-2021)
JHNM 2021, 31(1): 9-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ebrahimi S, Niknami M, Rafat F, Kazemnezhad Leili E. A Comparative Study on Adverse Pregnancy Outcomes in Pregnant Women with Different Age. JHNM 2021; 31 (1) :9-16
URL: http://hnmj.gums.ac.ir/article-1-1529-en.html
URL: http://hnmj.gums.ac.ir/article-1-1529-en.html
1- Midwifery (MSN), School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
2- Instructor, Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran ,niknamym@gmail.com
3- Instructor, Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
4- Associate Professor, Social Determinants of Health Research Center (SDHRC), Biostatistics, Guilan University of Medical Sciences, Rasht, Iran
2- Instructor, Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran ,
3- Instructor, Department of Midwifery, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
4- Associate Professor, Social Determinants of Health Research Center (SDHRC), Biostatistics, Guilan University of Medical Sciences, Rasht, Iran
Full-Text [PDF 527 kb]
(1008 Downloads)
| Abstract (HTML) (2702 Views)
Full-Text: (1173 Views)
Introduction
he pregnancy and childbirth are among the special events in women’s life and their families [1]. It is a period associated with physiological changes. If accompanied by risk factors, it can cause maternal death [2]. One of the risk factors for pregnancy is the mother’s age [3]. The women who are at reproductive age (19-35 years) face some problems that need to be addressed [4]. In modern societies, the number of women with delayed childbearing has greatly increased in recent decades [5]. In other words, the age of first pregnancy has now increased for many reasons. Factors such as long-term education, job preference, birth control by effective contraceptive methods, heavy working conditions, and economic problems have led to delay in pregnancy. Employment leads to high self-esteem, authority, and independence in women and changes in the family; therefore, the lack of maternal time reduces fertility and the desire for having more children [6].
According to the Centers for Disease Control (CDC) and Prevention, the proportion of first birth in women aged 35 years and older is about 8-fold higher and 15% of pregnancies after age of 35 years are now pregnant [4]. A woman with pregnancy after the age of 35 years can be worried about her reproductive age [7]. Pregnancy, especially for the first time at the age of 35 years, is considered as a high-risk pregnancy when the risk of complications increases, especially in older women with chronic illness or those who are physically weak, but the risk is lower in women with physical problem with no medical reason [4].
Another important age group in any community is adolescence [8]. According to the CDC report in 2015, 22.3% of newborns out of every 1000 are in this age group [9]. One of the reasons of adolescent pregnancy is its cultural and economic consequences such as poverty and illiteracy [10]. According to a report by the World Health Organization (WHO) on pregnancy in adolescents, age alone is not a risk factor; the education and social status are also effective [6]. The contradictory results in several studies have raised doubts about the consequences of pregnancy in adolescents and young adults. In Khalil et al.’s study, maternal age (>35 years) was significantly correlated with adverse pregnancy outcomes such as abortion, Small for Gestational Age (SGA), Large for Gestational Age (LGA), preeclampsia and cesarean section, compared with those with pregnancy at age< 35 years, but not with stillbirth, gestational hypertension, and spontaneous preterm delivery [11].
Another study reported no significant differences in distribution of mode of delivery, antenatal complications, cesarean section indications, perinatal mortality rate, and early neonatal complications between mothers aged <17 years and those aged 20-24 years [12]. In the study by Narukhutrpichai et al., women aged <20 years had a higher vaginal delivery rate despite observing a number of outcomes such as postpartum delivery and a 5-min Apgar score <7, and complications such as abnormal sedation, intrauterine growth restriction and postpartum hemorrhage were lower in them than in the age group of 20-34 years [13].
Given that the identification of disorders and complications caused by pregnancy at different ages can improve the treatment, and considering contradictory results in other countries and lack of related study in Iran, it is essential to increase community awareness of the pregnancy outcomes and provide proper care services to pregnant women. In this regard, this study aimed to determine pregnancy outcomes in pregnant women with different age.
Materials and Methods
This analytical study with a cross-sectional design was conducted on pregnant women referred to one of the maternity hospitals in Rasht, Iran from November 2017 to May 2018. The sample size was determined 345 considering 95% confidence level, 90% test power and distribution of pregnancy-induced hypertension (in adolescents)=0.057 reported by Zahiri et al. [14]. They were selected using sequential sampling method. The number required for each age group was also determined according to Zahiri et al. [14], as 69 in the age group <19 years, 138 in the age group 19-35 years, and 138 in the group >35 years. Pregnant women were followed from gestational age>37 weeks until delivery. Before their information was recorded, verbal informed consent was obtained from them. Inclusion criteria were: willingness to participate in the study, ability to have verbal communication and answer the questions.
In order to collect data, a two-part researcher-made questionnaire was used. The first part is for collecting demographic, fertility, and socioeconomic information through an interview. The second part surveys the adverse maternal and neonatal outcomes in pregnancy by checking maternal outcomes (gestational diabetes mellitus, preeclampsia, eclampsia, hypertension, preterm birth, post-term birth, multiple pregnancy, abnormal fetal presentation, shoulder dystocia, uterine rupture, uterine atony, fast childbirth, prolonged second pregnancy stage, active labor, placenta previa, placental abruption, recurrent abortion, late-term abortion, premature rupture of membranes, hydatidiform mole, ectopic pregnancy, anemia, and cesarean section) and neonatal outcomes (stillbirth, intrauterine growth retardation, low birthweight, macrosomia, neonatal resuscitation, 1- and 5-minute Apgar scores, meconium aspiration, congenital anomalies, fetal fracture, fetal death, RH in compatibility) through medical records. The history of pre-pregnancy disease was also surveyed by interviewing the mothers and observing their delivery records. To determine the validity of the instrument, the opinions of 10 faculty members of the Department of Obstetrics and Gynecology and the School of Nursing & Midwifery at Guilan University of Medical Sciences were used. Due to the nature of the instrument, there was no need to determine its reliability.
Questionnaires were completed when the condition of the mothers were stable. The answers to the adverse neonatal outcomes were given as “Yes” or “No”. In the end, the data were entered into the SPSS v. 21 software for analysis. Fisher’s exact test and chi-square test was to compare demographic, fertility, and socioeconomic variables; Kruskal-Wallis test, ANOVA, and Bonfrroni pot hoc test to compare adverse maternal and neonatal outcomes; Spearman correlation test to determine the correlation between variables; and multiple linear regression analysis to assess the relationship between maternal age and adverse maternal/neonatal outcomes. The P<0.05 was considered as significance level.
Results
The majority of mothers had a high school diploma (37.1%), and were housewives (94.5%) with a family income of 200-1250$ (55.4%). The majority had a history of infertility over 35 years (20.3%). The prevalence of unwanted/unexpected pregnancy was higher in the group <19 years (46.4%). The difference in infertility prevalence (P=0.001), participation in prenatal care classes (P=0.001) and vaccination (P=0.006) in three age groups were statistically significant. Among the adverse maternal outcomes, diabetes (P=0.001), abnormal fetal presentation (P=0.019), premature rupture of membranes (P=0.057, Borderline) and cesarean delivery (P=0.001) were significantly different between groups (Table 1), such that the prevalence of gestational diabetes was higher in the group >35 years (29.7%) and lower in the group under <19 years (0%).
.jpg)
Among the adverse neonatal outcomes, fetal death between three groups and intrauterine growth retardation between the groups <19 years and 19-35 years were significantly different (P<0.05). The prevalence of fetal death in the group <19 and >35 years old was 0%; it was reported only in the age group of 19-35 years (3.6%), which is significant according to Fisher’s exact test results (Table 2).

Comparing the prevalence of adverse maternal and neonatal outcomes in the three groups, results showed no statistically significant difference between the number of adverse neonatal outcomes and total adverse pregnancy outcomes, but the difference in the number of adverse maternal outcomes between the three groups was statistically significant (P=0.026). Based on Bonfrroni test results, the number of adverse maternal outcomes was significantly different between the age groups <19 and 19-35 years (P=0.024) and between the age groups <19 and >35 years (P=0.009). There was no significant difference in the number of adverse maternal outcomes between the two groups of 19-35 and >35 years.
Regarding the relationship between maternal age and maternal outcomes, multiple linear regression model showed that gestational age (P=0.002), participation in prenatal care classes (P=0.001), infertility (P=0.006), and mothers’ age (P=0.014) were the predictors of adverse maternal outcomes. Regarding the relationship between maternal age and neonatal outcomes, gestational age (P=0.001), infertility (P=0.001), participation in prenatal care classes (P=0.004) and mothers’ age in the age group <19 years (P=0.049) were the predictors of neonatal outcomes. Regarding the relationship between maternal age and overall pregnancy outcomes (Table 3), only gestational age (P=0.001), infertility (P=0.001), place of residence (P=0.007) and husband education had a significant relationship with overall pregnancy outcomes (P=0.01).
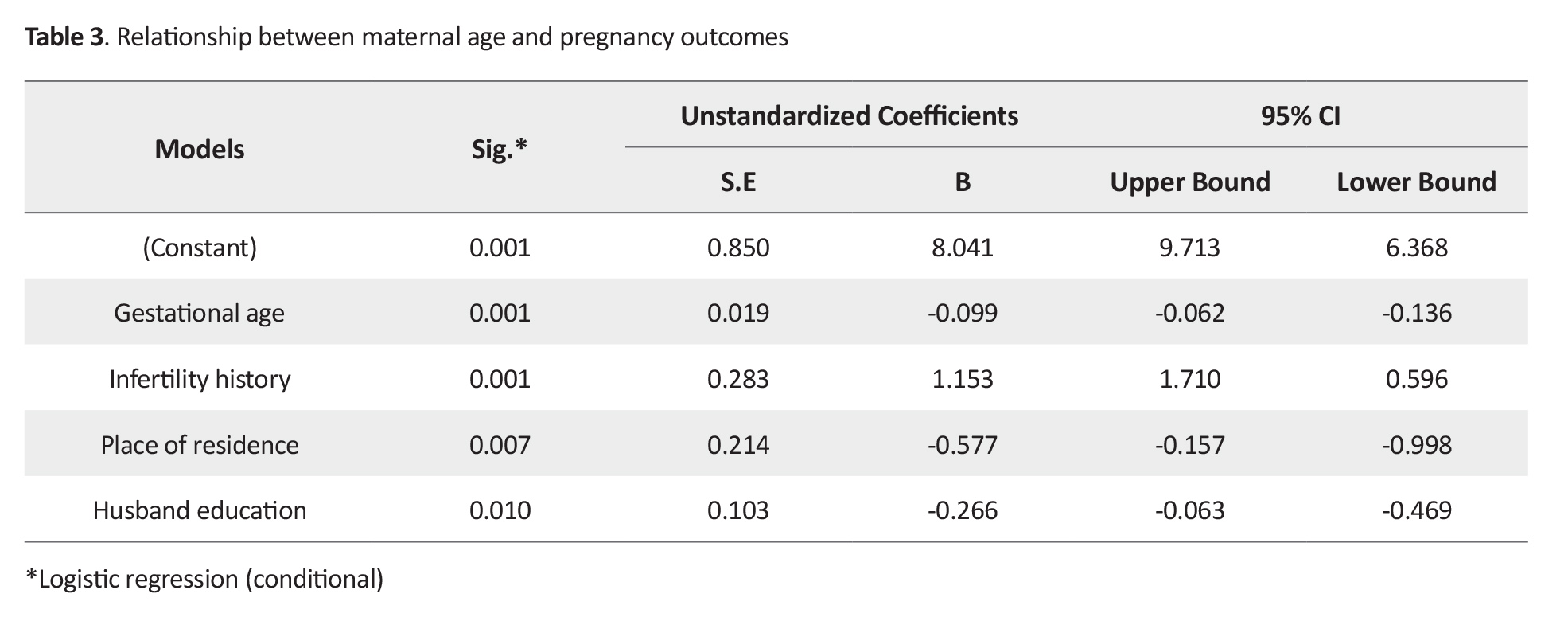
With the increase in husband’s educational level, the number of adverse pregnancy outcomes decreases (β=-0.266).
Discussion
This study aimed to determine the pregnancy outcomes in pregnant women with different age groups. The results showed that the highest percentage of maternal and neonatal outcomes were maternal diabetes, having cesarean section, and babies with birthweight <2500 g. Also, the percentage of maternal diabetes, abnormal fetal presentation, premature rupture of the membranes and the delivery by cesarean section were statistically significant in the studied groups. The prevalence of diabetes was increased with the increase of maternal age, which is consistent with the results of Aliyu et al. [15]. The highest prevalence of premature rupture of membranes was reported in pregnant mothers aged <19 years, which is also consistent with the results of Fadaei et al. [16].
The prevalence of cesarean section was higher in adolescent pregnant mothers, similar to other studies [17, 18] which can be due to the greater tendency of adolescent mothers to have vaginal delivery in this study. The highest frequency of anemia was seen in adolescent pregnant mothers. In the study by Pourali et al., there was no significant difference in the frequency of anemia between the two groups of adolescents and adults aged 20-35 years with, where it was higher in the adolescent group [19]. The need for iron supplementation in adolescent mothers significantly increases due to growth and menstruation, and iron deficiency in early adolescence causes anemia during pregnancy. Similar results have been reported in Gautam et al.’s study [20].
Consistent with our study, Figueredo et al. reported that the prevalence of preeclampsia was lower in adolescent pregnant mothers [21]. However, in other study, the prevalence of preeclampsia in teenage pregnant women was higher than in young pregnant women [22]. The possible reasons for this discrepancy are the different study area and the characteristics of subjects. In the study by Rezavand et al, abnormal fetal presentation between adolescent and young mothers was not significantly different [23] which may be why the prevalence of preterm labor and multiple pregnancy were higher in our study. In Aloufi et al.’s study, the prevalence of diabetes mellitus, cesarean section, and macrosomia in pregnant women aged >35 years was higher than that in those aged 20-35 years [24]. In our study, like the study by Lean et al., the prevalence of preeclampsia was also higher in mothers over 35 years of age [25].
Shafieian et al. found that the infants of adolescent mothers had significantly more complications than those of adult mothers, the most important complications of which was the intrauterine growth retardation [26]. In our study, the prevalence of intrauterine growth restriction was also higher in adolescent pregnant mothers. The fetus death rate in the group aged <19 years was zero indicating the lowest percentage of death rate is adolescent women, while in Pourali et al.’s study, the incidence of intrauterine death was higher in adult mothers and congenital anomalies and fetal death were higher in adolescent mothers [19].
The reason for this discrepancy may be the maternal and prenatal screening and lower underlying diseases in the adolescent mothers in our study. In the present study, the fetus death rate in mothers aged 19-35 years was higher, while in Dias et al.’s [27], the prevalence of fetus death was higher in the age group ³35 years. The reason for this discrepancy may be that the fetus death was followed up while they were in the hospital. Consistent with the present study, Ijarotimi et al. suggested that the prevalence of birth weight <2500 g in adults mothers was lower [28].
In our study, the prevalence of congenital anomalies in newborns were higher in mothers >35 years. In Olusanya et al.’s study, the number of anomalies in older mothers was also higher [29], but in Basirat et al.’s study, no congenital anomalies were reported in any age groups [30]. The reason for this discrepancy is probably the higher prevalence of diabetes in mothers over 35 years of age. There was a significant relationship between some of the maternal and neonatal outcomes. Pregnant women in these age groups are at high risk. It is, therefore, suggested to encourage women at high-risk to participate in childbirth preparation training courses during pregnancy.
Midwives have a great role in protecting the health of mothers. Therefore, further studies are recommended on reproductive organs and related factors (economic) using a larger sample size. One of the limitations of this study was the mothers’ psychological state after childbirth, which can affect their answers to the questions.
Ethical Considerations
Compliance with ethical guidelines
This paper was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1396.302).
Funding
This paper was extracted from the MSc. thesis of the first author in midwifery. It was supported by Social Determinents of Health Research Centers Guilan University of Medical Sciences.
Authors' contributions
Design, conceptualization, resources, and review: Soudabe Ebrahimi, Maryam Niknami, Fateme Rafat; Investigation, draft preparation and editing: Soudabe Ebrahimi, Maryam Niknami; Data collection: Soudabe Ebrahimi, Ehsan Kazemnezhad Leili; Data analysis: Soudabe Ebrahimi, Maryam Niknami, Ehsan Kazemnezhad Leili; Funding acquisition: All Authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy of Research of Guilan University of Medical Sciences and Social Determinents of Health Research Centers, Health Research Center in Guilan, and all the participants for their cooperation.
References
he pregnancy and childbirth are among the special events in women’s life and their families [1]. It is a period associated with physiological changes. If accompanied by risk factors, it can cause maternal death [2]. One of the risk factors for pregnancy is the mother’s age [3]. The women who are at reproductive age (19-35 years) face some problems that need to be addressed [4]. In modern societies, the number of women with delayed childbearing has greatly increased in recent decades [5]. In other words, the age of first pregnancy has now increased for many reasons. Factors such as long-term education, job preference, birth control by effective contraceptive methods, heavy working conditions, and economic problems have led to delay in pregnancy. Employment leads to high self-esteem, authority, and independence in women and changes in the family; therefore, the lack of maternal time reduces fertility and the desire for having more children [6].
According to the Centers for Disease Control (CDC) and Prevention, the proportion of first birth in women aged 35 years and older is about 8-fold higher and 15% of pregnancies after age of 35 years are now pregnant [4]. A woman with pregnancy after the age of 35 years can be worried about her reproductive age [7]. Pregnancy, especially for the first time at the age of 35 years, is considered as a high-risk pregnancy when the risk of complications increases, especially in older women with chronic illness or those who are physically weak, but the risk is lower in women with physical problem with no medical reason [4].
Another important age group in any community is adolescence [8]. According to the CDC report in 2015, 22.3% of newborns out of every 1000 are in this age group [9]. One of the reasons of adolescent pregnancy is its cultural and economic consequences such as poverty and illiteracy [10]. According to a report by the World Health Organization (WHO) on pregnancy in adolescents, age alone is not a risk factor; the education and social status are also effective [6]. The contradictory results in several studies have raised doubts about the consequences of pregnancy in adolescents and young adults. In Khalil et al.’s study, maternal age (>35 years) was significantly correlated with adverse pregnancy outcomes such as abortion, Small for Gestational Age (SGA), Large for Gestational Age (LGA), preeclampsia and cesarean section, compared with those with pregnancy at age< 35 years, but not with stillbirth, gestational hypertension, and spontaneous preterm delivery [11].
Another study reported no significant differences in distribution of mode of delivery, antenatal complications, cesarean section indications, perinatal mortality rate, and early neonatal complications between mothers aged <17 years and those aged 20-24 years [12]. In the study by Narukhutrpichai et al., women aged <20 years had a higher vaginal delivery rate despite observing a number of outcomes such as postpartum delivery and a 5-min Apgar score <7, and complications such as abnormal sedation, intrauterine growth restriction and postpartum hemorrhage were lower in them than in the age group of 20-34 years [13].
Given that the identification of disorders and complications caused by pregnancy at different ages can improve the treatment, and considering contradictory results in other countries and lack of related study in Iran, it is essential to increase community awareness of the pregnancy outcomes and provide proper care services to pregnant women. In this regard, this study aimed to determine pregnancy outcomes in pregnant women with different age.
Materials and Methods
This analytical study with a cross-sectional design was conducted on pregnant women referred to one of the maternity hospitals in Rasht, Iran from November 2017 to May 2018. The sample size was determined 345 considering 95% confidence level, 90% test power and distribution of pregnancy-induced hypertension (in adolescents)=0.057 reported by Zahiri et al. [14]. They were selected using sequential sampling method. The number required for each age group was also determined according to Zahiri et al. [14], as 69 in the age group <19 years, 138 in the age group 19-35 years, and 138 in the group >35 years. Pregnant women were followed from gestational age>37 weeks until delivery. Before their information was recorded, verbal informed consent was obtained from them. Inclusion criteria were: willingness to participate in the study, ability to have verbal communication and answer the questions.
In order to collect data, a two-part researcher-made questionnaire was used. The first part is for collecting demographic, fertility, and socioeconomic information through an interview. The second part surveys the adverse maternal and neonatal outcomes in pregnancy by checking maternal outcomes (gestational diabetes mellitus, preeclampsia, eclampsia, hypertension, preterm birth, post-term birth, multiple pregnancy, abnormal fetal presentation, shoulder dystocia, uterine rupture, uterine atony, fast childbirth, prolonged second pregnancy stage, active labor, placenta previa, placental abruption, recurrent abortion, late-term abortion, premature rupture of membranes, hydatidiform mole, ectopic pregnancy, anemia, and cesarean section) and neonatal outcomes (stillbirth, intrauterine growth retardation, low birthweight, macrosomia, neonatal resuscitation, 1- and 5-minute Apgar scores, meconium aspiration, congenital anomalies, fetal fracture, fetal death, RH in compatibility) through medical records. The history of pre-pregnancy disease was also surveyed by interviewing the mothers and observing their delivery records. To determine the validity of the instrument, the opinions of 10 faculty members of the Department of Obstetrics and Gynecology and the School of Nursing & Midwifery at Guilan University of Medical Sciences were used. Due to the nature of the instrument, there was no need to determine its reliability.
Questionnaires were completed when the condition of the mothers were stable. The answers to the adverse neonatal outcomes were given as “Yes” or “No”. In the end, the data were entered into the SPSS v. 21 software for analysis. Fisher’s exact test and chi-square test was to compare demographic, fertility, and socioeconomic variables; Kruskal-Wallis test, ANOVA, and Bonfrroni pot hoc test to compare adverse maternal and neonatal outcomes; Spearman correlation test to determine the correlation between variables; and multiple linear regression analysis to assess the relationship between maternal age and adverse maternal/neonatal outcomes. The P<0.05 was considered as significance level.
Results
The majority of mothers had a high school diploma (37.1%), and were housewives (94.5%) with a family income of 200-1250$ (55.4%). The majority had a history of infertility over 35 years (20.3%). The prevalence of unwanted/unexpected pregnancy was higher in the group <19 years (46.4%). The difference in infertility prevalence (P=0.001), participation in prenatal care classes (P=0.001) and vaccination (P=0.006) in three age groups were statistically significant. Among the adverse maternal outcomes, diabetes (P=0.001), abnormal fetal presentation (P=0.019), premature rupture of membranes (P=0.057, Borderline) and cesarean delivery (P=0.001) were significantly different between groups (Table 1), such that the prevalence of gestational diabetes was higher in the group >35 years (29.7%) and lower in the group under <19 years (0%).
.jpg)
Among the adverse neonatal outcomes, fetal death between three groups and intrauterine growth retardation between the groups <19 years and 19-35 years were significantly different (P<0.05). The prevalence of fetal death in the group <19 and >35 years old was 0%; it was reported only in the age group of 19-35 years (3.6%), which is significant according to Fisher’s exact test results (Table 2).

Comparing the prevalence of adverse maternal and neonatal outcomes in the three groups, results showed no statistically significant difference between the number of adverse neonatal outcomes and total adverse pregnancy outcomes, but the difference in the number of adverse maternal outcomes between the three groups was statistically significant (P=0.026). Based on Bonfrroni test results, the number of adverse maternal outcomes was significantly different between the age groups <19 and 19-35 years (P=0.024) and between the age groups <19 and >35 years (P=0.009). There was no significant difference in the number of adverse maternal outcomes between the two groups of 19-35 and >35 years.
Regarding the relationship between maternal age and maternal outcomes, multiple linear regression model showed that gestational age (P=0.002), participation in prenatal care classes (P=0.001), infertility (P=0.006), and mothers’ age (P=0.014) were the predictors of adverse maternal outcomes. Regarding the relationship between maternal age and neonatal outcomes, gestational age (P=0.001), infertility (P=0.001), participation in prenatal care classes (P=0.004) and mothers’ age in the age group <19 years (P=0.049) were the predictors of neonatal outcomes. Regarding the relationship between maternal age and overall pregnancy outcomes (Table 3), only gestational age (P=0.001), infertility (P=0.001), place of residence (P=0.007) and husband education had a significant relationship with overall pregnancy outcomes (P=0.01).

With the increase in husband’s educational level, the number of adverse pregnancy outcomes decreases (β=-0.266).
Discussion
This study aimed to determine the pregnancy outcomes in pregnant women with different age groups. The results showed that the highest percentage of maternal and neonatal outcomes were maternal diabetes, having cesarean section, and babies with birthweight <2500 g. Also, the percentage of maternal diabetes, abnormal fetal presentation, premature rupture of the membranes and the delivery by cesarean section were statistically significant in the studied groups. The prevalence of diabetes was increased with the increase of maternal age, which is consistent with the results of Aliyu et al. [15]. The highest prevalence of premature rupture of membranes was reported in pregnant mothers aged <19 years, which is also consistent with the results of Fadaei et al. [16].
The prevalence of cesarean section was higher in adolescent pregnant mothers, similar to other studies [17, 18] which can be due to the greater tendency of adolescent mothers to have vaginal delivery in this study. The highest frequency of anemia was seen in adolescent pregnant mothers. In the study by Pourali et al., there was no significant difference in the frequency of anemia between the two groups of adolescents and adults aged 20-35 years with, where it was higher in the adolescent group [19]. The need for iron supplementation in adolescent mothers significantly increases due to growth and menstruation, and iron deficiency in early adolescence causes anemia during pregnancy. Similar results have been reported in Gautam et al.’s study [20].
Consistent with our study, Figueredo et al. reported that the prevalence of preeclampsia was lower in adolescent pregnant mothers [21]. However, in other study, the prevalence of preeclampsia in teenage pregnant women was higher than in young pregnant women [22]. The possible reasons for this discrepancy are the different study area and the characteristics of subjects. In the study by Rezavand et al, abnormal fetal presentation between adolescent and young mothers was not significantly different [23] which may be why the prevalence of preterm labor and multiple pregnancy were higher in our study. In Aloufi et al.’s study, the prevalence of diabetes mellitus, cesarean section, and macrosomia in pregnant women aged >35 years was higher than that in those aged 20-35 years [24]. In our study, like the study by Lean et al., the prevalence of preeclampsia was also higher in mothers over 35 years of age [25].
Shafieian et al. found that the infants of adolescent mothers had significantly more complications than those of adult mothers, the most important complications of which was the intrauterine growth retardation [26]. In our study, the prevalence of intrauterine growth restriction was also higher in adolescent pregnant mothers. The fetus death rate in the group aged <19 years was zero indicating the lowest percentage of death rate is adolescent women, while in Pourali et al.’s study, the incidence of intrauterine death was higher in adult mothers and congenital anomalies and fetal death were higher in adolescent mothers [19].
The reason for this discrepancy may be the maternal and prenatal screening and lower underlying diseases in the adolescent mothers in our study. In the present study, the fetus death rate in mothers aged 19-35 years was higher, while in Dias et al.’s [27], the prevalence of fetus death was higher in the age group ³35 years. The reason for this discrepancy may be that the fetus death was followed up while they were in the hospital. Consistent with the present study, Ijarotimi et al. suggested that the prevalence of birth weight <2500 g in adults mothers was lower [28].
In our study, the prevalence of congenital anomalies in newborns were higher in mothers >35 years. In Olusanya et al.’s study, the number of anomalies in older mothers was also higher [29], but in Basirat et al.’s study, no congenital anomalies were reported in any age groups [30]. The reason for this discrepancy is probably the higher prevalence of diabetes in mothers over 35 years of age. There was a significant relationship between some of the maternal and neonatal outcomes. Pregnant women in these age groups are at high risk. It is, therefore, suggested to encourage women at high-risk to participate in childbirth preparation training courses during pregnancy.
Midwives have a great role in protecting the health of mothers. Therefore, further studies are recommended on reproductive organs and related factors (economic) using a larger sample size. One of the limitations of this study was the mothers’ psychological state after childbirth, which can affect their answers to the questions.
Ethical Considerations
Compliance with ethical guidelines
This paper was approved by the Ethics Committee of Guilan University of Medical Sciences (Code: IR.GUMS.REC.1396.302).
Funding
This paper was extracted from the MSc. thesis of the first author in midwifery. It was supported by Social Determinents of Health Research Centers Guilan University of Medical Sciences.
Authors' contributions
Design, conceptualization, resources, and review: Soudabe Ebrahimi, Maryam Niknami, Fateme Rafat; Investigation, draft preparation and editing: Soudabe Ebrahimi, Maryam Niknami; Data collection: Soudabe Ebrahimi, Ehsan Kazemnezhad Leili; Data analysis: Soudabe Ebrahimi, Maryam Niknami, Ehsan Kazemnezhad Leili; Funding acquisition: All Authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Deputy of Research of Guilan University of Medical Sciences and Social Determinents of Health Research Centers, Health Research Center in Guilan, and all the participants for their cooperation.
References
- Kamali Fard M, Alizadeh R, Sehati Shafaei F, Gojazadeh M. [The effect of lifestyle on the rate of preterm birth (Persian)]. Journal of Ardabil University of Medical Sciences. 2010; 10(1):55-63. http://jarums.arums.ac.ir/article-1-235-en.html
- James D, Steer P, Weiner C, Gonik B, Robson S. High-risk pregnancy: Management options. Cambridge: Cambridge University Press; 2017. [DOI:10.1017/9781108349185]
- Benli AR, Benli NC, Usta AT, Atakul T, Koroglu M. Effect of maternal age on pregnancy outcome and cesarean delivery rate. Journal of Clinical Medicine Research. 2015; 7(2):97-102. [DOI:10.14740/jocmr1904w] [PMID] [PMCID]
- Cunningham FG, Leveno KJ, Bloom SL, Rouse D, Raniney B, Hauth JC, et al. Williams obstetrics. New York: McGraw Hill. 24th edition; 2014. https://books.google.com/books?id=XPe4kgEACAAJ&dq
- Balasch U, Gratacós E. Delayed childbearing: Effects on fertility and the outcome of pregnancy. Fetal Diagnosis and Therapy. 2011; 29(4):263-73. [DOI:10.1159/000323142] [PMID]
- Abbas AM, Ali SS, Ali MK, Fouly H, Altraigey A. The maternal and neonatal outcomes of teenage pregnancy in a tertiary university hospital in Egypt. Proceedings in Obstetrics and Gynecology. 2017; 7(3):1-10. [DOI:10.17077/2154-4751.1350]
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final data for 2015. National Vital Statistics Reports. 2017; 66(1):1. [PMID]
- Rowlands S. Social predictors of repeat adolescent pregnancy and focussed strategies. Best Practice & Research Clinical Obstetrics & Gynaecology. 2010; 24(5):605-16. [DOI:10.1016/j.bpobgyn.2010.02.016] [PMID]
- Simbor M. [Adolescent reproductive health (Persian)]. Golban: Tehran; 2015. p. 57-59. http://opac.nlai.ir/opac-prod/bibliographic/3467983
- Varney H, Kriebs JM, Gegor CL. [Z. Taghizadeh Z, M. Geranmayeh, F. Vasegh Rahimpour, Persian trans.]. Varney's midwifery. Tehran: Andisheh Rafie; 2009. p. 103-105. http://opac.nlai.ir/opac-prod/bibliographic/1202154
- Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound in Obstetrics & Gynecology. 2013; 42(6):634-43. [DOI:10.1002/uog.12494] [PMID]
- Abu-Heija A, Ali AM, Al-Dakheil S. Obstetrics and perinatal outcome of adolescent nulliparous pregnant women. Gynecologic and Obstetric Investigation. 2002; 53(2):90-2. [DOI:10.1159/000053000] [PMID]
- Narukhutrpichai P, Khrutmuang D, Chattrapiban T. The obstetrics and neonatal outcomes of teenage pregnancy in Naresuan University hospital. Journal of the Medical Association of Thailand. 2016; 99(4):361-7. [PMID]
- Zahiri Z, Sharami SH, Faraji R, Asghar Nia M, Atrkar Roshan Z. [Survey the association between maternal age and pregnancy outcome (Persian)]. Journal of Guilan University of Medical Sciences. 2007; 15(60):73-8. http://journal.gums.ac.ir/article-1-433-en.html
- Aliyu MH, Luke S, Kristensen S, Alio AP, Salihu HM. Joint effect of obesity and teenage pregnancy on the risk of preeclampsia: A population-based study. The Journal of Adolescent Health. 2010; 46(1):77-82. [DOI:10.1016/j.jadohealth.2009.06.006] [PMID]
- Fadaei B, Movahedi M, Akbari M, Ghasemi M, Jalalvand A. [Effect of maternal age on pregnancy outcome (Persian)]. Journal of Isfahan Medical School. 2011; 29(145):855-60. http://jims.mui.ac.ir/index.php/jims/article/view/1066
- Konje JC, Palmer A, Waston A, Hay DM, Imrie A, Ewings P. Early teenage pregnancies in Hull. British Journal of Obstetrics and Gynaecology. 1992; 99(12):969-73. [DOI:10.1111/j.1471-0528.1992.tb13699.x] [PMID]
- Kaźmierczak W, Fiegler-Rudol P, Wegrzyn P, Cholewa D, Kliś J. [Pregnancy complications and pregnancy outcome in women under 18 and above 35 years old in highly industrialized urban complex of Upper Silesia (Polish)]. Ginekologia Polska. 2005; 76(12):980-5. [PMID]
- Poorali L, Ayati S, Shakeri MT, Vatanchi A, Khatami Sh. [Obstetric and perinatal outcomes in primigravid adolescent and adult women (Persian)]. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2018; 21(4):1-7. [DOI:10.22038/ijogi.2018.11214]
- Gautam S, Min H, Kim H, Jeong HS. Determining factors for the prevalence of anemia in women of reproductive agein Nepal: Evidence from recent national survey data. PLoS One. 2019; 14(6):e0218288. [DOI:10.1371/journal.pone.0218288] [PMID] [PMCID]
- Figuerêdo ED, Lamy Filho F, Lamy ZC, da Silva AAM. Maternal age and adverse perinatal outcomes in a birth cohort (BRISA) from a Northeastern Brazilian city. Revista Brasileira de Ginecologia e Obstetrícia. 2014; 36(12):562-8. [DOI:10.1590/SO100-720320140005161] [PMID]
- Talawar S, Venkatesh G. Outcome of teenage pregnancy. IOSR Journal of Dental and Medical Sciences. 2013; 6(6):81-3. [DOI:10.9790/0853-0668183]
- Rezavand N, Zangeneh M, Malek Khosravi SH, Rezaee M. [A comparative study of pregnancy results in adolescents and young mothers referred to the Motazedi Hospital in Kermanshah (Persian)]. Nursing And Midwifery Journal. 2009; 7(3):136-41. http://unmf.umsu.ac.ir/article-1-103-en.html
- Aloufi W, Othman M , Aloufi Sh, Alharbi S, Aboqarn L, Ahmed A, et al. Impact of maternal age on pregnancy in madinah region. EC Gynecology. 2019; 8(10):984-90. https://www.ecronicon.com/ecgy/pdf/ECGY-08-00417.pdf
- Lean SC, Derricott H, Jones RL, Heazel AEP. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS One. 2017; 12(10):e0186287. [DOI:10.1371/journal.pone.0186287] [PMID] [PMCID]
- Shafieian M, Bhadoran P, Amini R, Amini Y, Jafarpour M, Hematian A. [Social, economical and health outcomes of pregnancy in young adults: A review article (Persian)]. Journal of Ilam University of Medical Sciences. 2014; 22(3):34-40. http://sjimu.medilam.ac.ir/article-1-1944-en.html
- Dias T, Wijesinghe E, Abeykoon S, Ganeshamoorthy P, Kumarasiri S, Kodithuwakku M, et al. Pregnancy outcome in extremes of reproductive age at a tertiary care hospital. Sri Lanka Journal of Obstetrics and Gynaecology. 2013; 35(3):77-9. [DOI:10.4038/sljog.v35i3.6334]
- Ijarotimi OA, Biobaku OR, Badejoko OO, Loto OM, Orji EO. Obstetric outcome of teenage pregnancy and labour in Obafemi Awolowo University Teaching Hospitals complex, Ile-Ife: A ten year review. Tropical Journal of Obstetrics and Gynaecology. 2019; 36(1):105‑11. [DOI:10.4103/TJOG.TJOG_13_19]
- Olusanya BO, Solanke OA. Perinatal correlates of delayed childbearing in a developing country. Archives of Gynecology and Obstetrics. 2012; 285(4):951-7. [DOI:10.1007/s00404-011-2105-5] [PMID]
- Basirat Z, Haji Ahmadi M. [Comparison of the frequency of pregnancy complications before and after the age of 35 years old (Persian)]. Journal of Babol University of Medical Sciences. 2003; 5(3):35-9. http://jbums.org/article-1-2760-en.html
Article Type : Research |
Subject:
General
Received: 2020/12/19 | Accepted: 2021/12/29 | Published: 2021/12/29
Received: 2020/12/19 | Accepted: 2021/12/29 | Published: 2021/12/29
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






